将炔酸的三官能化、烯原位 C-H 官能化和胺化结合起来:非对称 2,3-二芳基取代吲哚的制备方法
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报告了一种同时构建两个 C-C 键和两个 C-N 键的统一方法,该方法结合了炔酸三官能化、正交 C-H 官能化和胺化级联反应。在有序过程中,区域选择性炔插入反应比脱羧过程更有利。炔酸中羧基的存在确保了羰基钯化过程的高区域选择性,为合成具有优异区域选择性的非对称 2,3 二芳基取代吲哚支架的新方法铺平了道路。实验证明该方法适用于克级合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
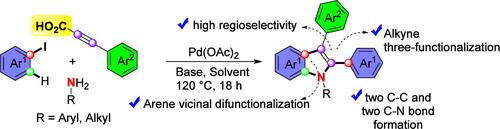
Combining Trifunctionalization of Alkynoic Acids, Arene ortho C–H Functionalization and Amination: An Approach to Unsymmetrical 2,3-Diaryl Substituted Indoles
Here we report a simultaneous construction of two C–C and two C–N bonds in a unified procedure that incorporates alkynoic acid trifunctionalization, ortho C–H functionalization, and amination cascade. In an ordered process, the regioselective alkyne insertion reaction is favored over the decarboxylation process. The presence of the carboxyl group in alkynoic acid ensures the high regioselectivity in the carbopalladation process, paving the way for a novel method to synthesize unsymmetrically 2,3-diaryl substituted indole scaffolds with excellent regioselectivity. The protocol is demonstrated to be suitable for gram-scale synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


