m6Am 可封存 PCF11,从而抑制过早终止并驱动神经母细胞瘤分化
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
N6,2′-O-二甲基腺苷(m6Am)是一种影响多种疾病的大量 mRNA 修饰,但由于 m6Am 的阅读器尚不清楚,其功能仍存在争议。利用定量蛋白质组学,我们发现转录终止子过早裂解因子 II(PCF11)是人体细胞中 m6Am 的特异性阅读器。成熟 RNA 与新生 RNA 的直接定量分析显示,m6Am 并不调节 mRNA 的稳定性,而是促进新生转录。从机理上讲,m6Am 通过将 PCF11 与近端 RNA 聚合酶 II(RNA Pol II)隔离而发挥作用。m6Am 与 PCF11 的独特关系意味着,当 PCF11 减少时,m6Am 的功能就会增强,这种情况发生在全反式维甲酸(ATRA)诱导的神经母细胞瘤分化治疗过程中。在这里,m6Am 可促进 ATF3 的表达,而 ATF3 可抑制神经母细胞瘤生物标志物 MYCN。消耗m6Am可抑制ATRA处理的神经母细胞瘤的MYCN抑制,并保持其肿瘤干样特性。总之,我们将 m6Am 定性为一种抗终止子 RNA 修饰,它能抑制过早终止并调节神经母细胞瘤的治疗反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
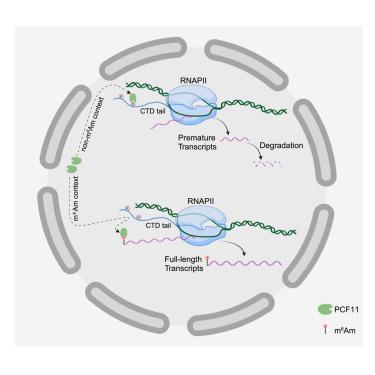
m6Am sequesters PCF11 to suppress premature termination and drive neuroblastoma differentiation
N6,2′-O-dimethyladenosine (m6Am) is an abundant mRNA modification that impacts multiple diseases, but its function remains controversial because the m6Am reader is unknown. Using quantitative proteomics, we identified transcriptional terminator premature cleavage factor II (PCF11) as a m6Am-specific reader in human cells. Direct quantification of mature versus nascent RNAs reveals that m6Am does not regulate mRNA stability but promotes nascent transcription. Mechanistically, m6Am functions by sequestering PCF11 away from proximal RNA polymerase II (RNA Pol II). This suppresses PCF11 from dissociating RNA Pol II near transcription start sites, thereby promoting full-length transcription of m6Am-modified RNAs. m6Am’s unique relationship with PCF11 means m6Am function is enhanced when PCF11 is reduced, which occurs during all-trans-retinoic-acid (ATRA)-induced neuroblastoma-differentiation therapy. Here, m6Am promotes expression of ATF3, which represses neuroblastoma biomarker MYCN. Depleting m6Am suppresses MYCN repression in ATRA-treated neuroblastoma and maintains their tumor-stem-like properties. Collectively, we characterize m6Am as an anti-terminator RNA modification that suppresses premature termination and modulates neuroblastoma’s therapeutic response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


