用计算和实验综合方法鉴定新的木瓜蛋白酶抑制剂
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
SARS-CoV-2 木瓜蛋白酶(PLpro)是一种关键的病毒蛋白酶,在病毒复制和致病过程中起着至关重要的作用,因此是开发抗病毒疗法的一个有吸引力的靶点。因此,基于绿原酸作为一种对病毒蛋白酶具有抑制特性的天然化合物的公认特性,我们选择了绿原酸作为 PLpro 的靶标。研究策略包括体内和体外技术的协同组合,从而成功鉴定了 PLpro 的天然抑制剂。在对接分析中观察到,与 GRL0617 相比,绿原酸与 PLpro 的相互作用能更为明显。此外,分子动力学模拟表明,绿原酸-PLpro 复合物具有显著的稳定性,而且在构象动力学上非常接近。后处理终态自由能评估表明,两种分子的亲和力相当。此外,为进行剂量反应分析而进行的酶抑制试验验证了绿原酸是 PLpro 的潜在抑制剂。这一实验评估加强了支持绿原酸对 PLpro 抑制活性的证据。总之,这项综合分析为开发针对 PLpro 的潜在候选疗法提供了一个宝贵的平台,为抗击 SARS-CoV-2 提供了广阔的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
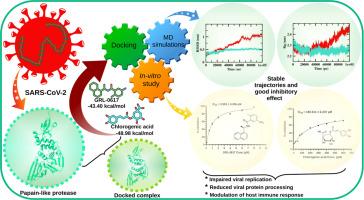
Integrated computational and experimental approaches to identify new papain-like protease inhibitors
SARS-CoV-2 papain-like protease (PLpro), a key viral protease, plays a crucial role in the viral replication and pathogenesis, making it an attractive target for the development of antiviral therapies. Thus to target PLpro, the chlorogenic acid was chosen based on its well-established characteristics as a natural compound with inhibitory properties against viral proteases. The research strategy involved a synergistic combination of in-silico and in-vitro techniques, enabled successful identification of a natural inhibitor for PLpro. In the docking analysis, it was observed that chlorogenic acid exhibited a more pronounced interaction energy with PLpro compared to GRL0617. Additionally, the molecular dynamics simulations demonstrated remarkable stability of the chlorogenic acid-PLpro complex, along with close proximity in conformational dynamics. The assessment of post-processing end-state free energies indicated comparable affinities for both molecules. Furthermore, the enzymatic inhibition assay performed for dose-response analysis provided validation for chlorogenic acid as a potential inhibitor of PLpro. This experimental assessment strengthens the evidence supporting the inhibitory activity of chlorogenic acid against PLpro. Overall, this comprehensive analysis serves as a valuable platform for the development of potential therapeutic candidates targeting PLpro, offering promising prospects for combating SARS-CoV-2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


