作为强抗氧化剂衍生物的曲酸槲皮素类似物:理论见解
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
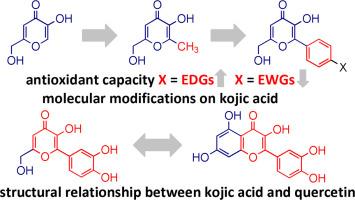
曲酸是一种由多种真菌产生的天然产品,具有广泛的用途。其螯合和抗氧化能力与其化学和生物特性有关。本研究提出了一些分子修饰方法,并在曲酸的基本结构中引入了取代苯基和羟基苯基。抗氧化能力由 DFT/B3LYP/6-311++G(2d,2p) 计算得出。在气相和 PCM 方法中,考虑了不同的抗氧化机制,如前沿分子轨道(HOMO、LUMO 和 GAP)、电子(IP、SET 和 SPLET)或氢转移(BDEOH 和 HAT)。在所有研究机制中,苯基都能提高抗氧化能力,尤其是当苯基环的对位存在电子供体(EDG)时,与电子撤回基团(EWG)相比。化学稳定性与阳离子自由基和半醌的自旋密度贡献相一致。在气相和水中观察到曲酸和槲皮素的羟基衍生物结构相似。与烯醇位置相比,苯酚位置上较低的 BDEOH 值对抗氧化能力更为重要。酚类半醌比烯醇类更稳定。化学稳定性取决于共振结构的数量和未配对电子贡献率最高的位置。总之,苯基取代是提高曲酸抗氧化能力的重要分子修饰方法。槲皮素类似物被认为是曲酸的强抗氧化衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quercetin analogues of kojic acid as strong antioxidant derivatives: Theoretical insights
Kojic acid is a natural product produced by many fungal species and has a wide range of applications. The chelating and antioxidant capacity are associated with their chemical and biological properties. In this study, some molecular modifications were proposed and substituted and hydroxylated phenyl moieties were introduced in the basic structure of kojic acid. The antioxidant capacities were calculated by DFT/B3LYP/6-311++G(2d,2p). Different antioxidant mechanisms were considered such as frontier molecular orbitals (HOMO, LUMO, and GAP), electron (IP, SET, and SPLET), or hydrogen transfers (BDEOH and HAT) on gas phase and PCM methods. The phenyl increases the antioxidant capacity, especially when an electron donating group (EDG) is found at the para position of the phenyl ring when compared to electron withdrawing groups (EWGs), for all studied mechanisms. The chemical stability agrees with spin density contributions for their cationic free radicals and semiquinones. A structural similarity between hydroxylated derivatives of kojic acid and quercetin was observed on gas phase and water. The lower BDEOH values at the phenol positions are more important for the antioxidant capacity than at the enol position. Phenolic semiquinones are more stables than enolic ones. The chemical stability depends on the number of resonance structures and the positions where the unpaired electron can be found with the highest contributions. In conclusion, phenyl substitution is a valuable molecular modification for the increase on antioxidant capacity of kojic acid. Quercetin analogues of kojic acid were proposed as strong antioxidant derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


