带有氟甲基喹啉取代基的新型酞菁:合成、表征、光物理和光化学特性
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
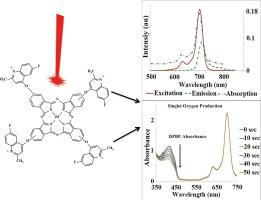
制备了新型 4-((6-氟-2-甲基喹啉-4-基)氧基)酞腈 (3a)、3-((6-氟-2-甲基喹啉-4-基)氧基)酞腈 (3b)、四 6-氟-2-甲基喹啉-4-基)氧基取代的锌(II)酞菁 (4a 和 4b)及其水溶性衍生物 (5a 和 5b)。通过傅立叶变换红外光谱(FT-IR)、1HNMR、紫外可见光谱(UV-Vis)和 MALDI-TOF 质量数据证实了这些新型化合物的结构。为了研究外围四取代锌(II)酞菁(4a 和 4b)和水溶性季铵化四取代锌(II)酞菁(5a 和 5b)的光动力治疗潜力,对它们的聚集倾向、荧光量子产率、单线态氧量子产率和光降解进行了测定。研究结果表明,新型四-6-氟-2-甲基喹啉-4-基)氧取代锌(II)酞菁(4a 和 4b)及其水溶性衍生物(5a 和 5b)由于没有聚集倾向、有足够的荧光发射用于监测和产生大量的单线态氧以破坏癌组织以及具有适度的光稳定性,可用作光敏剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel phthalocyanines bearing fluoro-methylquinolin substituents: Synthesis, characterization, photophysical and photochemical properties
The novel 4-((6-fluoro-2-methylquinolin-4-yl)oxy)phthalonitrile (3a), 3-((6-fluoro-2-methylquinolin-4-yl)oxy)phthalonitrile (3b), tetra 6-fluoro-2-methylquinolin-4-yl)oxy substituted zinc(II) phthalocyanines (4a and 4b) and their water soluble derivatives (5a and 5b) were prepared. The proposed structures of novel compounds were comfirmed via FT-IR, 1HNMR , UV–Vis and MALDI-TOF mass data. The aggregation tendency, fluorescence quantum yield, singlet oxygen quantum yield and photodegradation measurements were performed to examine the photodynamic therapy potential of both peripherally tetra substituted zinc(II) phthalocyanines (4a and 4b) and the water soluble quaternized tetra substituted zinc(II) phthalocyanines (5a and 5b). The result showed that both the novel tetra 6-fluoro-2-methylquinolin-4-yl)oxy substituted zinc(II) phthalocyanines (4a and 4b) and their water soluble derivatives (5a and 5b) could be used as photosensitizer agents in PDT thanks to their lack of aggregation tendency, having sufficient fluorescence emission for monitoring and produce high amount of singlet oxygen to destroy cancerous tissues and having moderate photostability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


