设计二茂铁基噻吩查耳酮作为染料敏化太阳能电池的候选光收集器
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
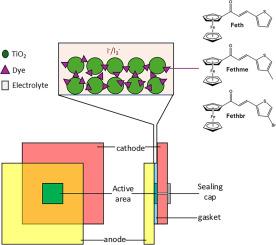
在染料敏化太阳能电池(DSSC)中,染料(或光敏剂)起着至关重要的作用。它吸收光并产生电子,从而影响将太阳光转化为电能的效率。虽然基于 Ru(II) 的染料在 DSSC 中很常见,但它们的稀缺性、易降解性和有限的吸收范围给广泛应用带来了挑战。我们合成了三种新的二茂铁基噻吩化合物,它们都具有相同的核心结构,但明显不同的是噻吩环上存在甲基(CH3)和溴(Br)取代基。利用光谱和 X 射线晶体学分析获得的结构,对这些化合物的化学反应性进行了理论评估。循环伏安法(CV)分析和电化学阻抗光谱法(EIS)被用来研究材料的氧化还原特性和电子传输机制。溴化过程证明了其在染料应用方面的功效,而噻吩环上的甲基附着增强了对 TiO₂的锚定,有助于提高 DSSC 的性能。总体而言,与其他化合物相比,含有溴的化合物具有更低的带隙,因此在太阳能模拟分析中具有更高的效率,几乎是含有甲基的化合物的两倍,并大大超过了普通的噻吩化合物。EIS 分析表明,在三种二茂铁基查尔酮染料中,含溴化合物的电荷重组阻力最大,电子寿命最长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Designing ferrocenyl thiophene chalcones as light harvester candidates for dye-sensitized solar cells
In dye-sensitized solar cells (DSSCs), the dye (or photosensitizer) plays a crucial role. It absorbs light and generates electrons, which affects the efficiency of converting sunlight into electricity. While Ru(II)-based dyes are common in DSSCs, their scarcity, susceptibility to degradation, and limited absorption range pose challenges for wider adoption. Three new ferrocenyl-thiophene compounds have been synthesized, all sharing the same core structure, but the distinctive difference is the existence of methyl group (CH3) and bromine (Br) substituents attached to the thiophene ring. Using the structures obtained from spectroscopic and X-ray crystallography analyzes, the chemical reactivity of these compounds is theoretically evaluated. Cyclic voltammetry (CV) analysis and electrochemical impedance spectroscopy (EIS) were employed to investigate the redox properties and electron transport mechanisms of the material. The bromination process demonstrates its efficacy for dye applications, while the methyl attachment to the thiophene ring enhances anchoring toward TiO₂, contributing to improved performance in DSSCs. Overall, the compound featuring bromine exhibited a lower band gap compared to the others, resulting in higher efficiency in solar simulation analysis, nearly double that of the methyl-containing compound, and significantly surpassing the plain thiophene compound. EIS analysis revealed that, among the three ferrocenyl chalcone dyes, the bromine-containing compound exhibited the highest charge recombination resistance and longest electron lifetime.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


