RuIII(pic)3 复合物与半胱氨酸的氧化还原反应:光谱、动力学和生物学研究
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
采用停流和快速扫描分光光度法研究了[RuIII(pic)3](pic- = picolinate)与半胱氨酸还原形成相应的红色[RuII(pic)3]-复合物(λmax = 466 nm,εmax = 12,000 M-1 cm-1)的动力学。在 466 纳米波长处跟踪了反应的时间进程与半胱氨酸浓度、pH 值和温度的函数关系。动力学数据(k = 291 ± 4 M-1 s-1,温度为 25 °C,pH 值为 8.4)和活化参数(ΔH≠ = 36 ± 1 kJ mol-1 和 ΔS≠ = -78 ± 3 J mol-1 deg-1)被解释为涉及 Ru(III)和半胱氨酸之间决定速率的外层电子转移机制。研究还考察了[RuIII(pic)3]复合物在无半胱氨酸和有半胱氨酸存在的情况下对细菌(肺炎克雷伯氏菌和金黄色葡萄球菌)生长的抑制作用,结果表明添加半胱氨酸对该复合物对金黄色葡萄球菌的抗菌活性有积极影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
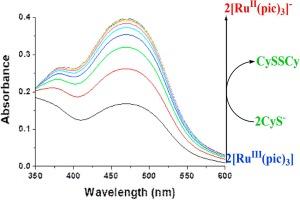
Redox reaction of a RuIII(pic)3 complex with cysteine: Spectral, kinetic and biological studies
The kinetics of the reduction of [RuIII(pic)3] (pic− = picolinate) with cysteine leading to the formation of the corresponding red coloured [RuII(pic)3]− complex (λmax = 466 nm, εmax = 12,000 M−1 cm−1) was studied by using stopped-flow and rapid scan spectrophotometry. The time course of the reaction was followed at 466 nm as a function of cysteine concentration, pH and temperature. Kinetic data (k = 291 ± 4 M−1 s−1 at 25 °C and pH 8.4) and activation parameters (ΔH≠ = 36 ± 1 kJ mol−1 and ΔS≠ = −78 ± 3 J mol−1 deg−1) are interpreted in terms of a mechanism involving rate-determining outer-sphere electron transfer between Ru(III) and the cysteine. Inhibition of bacterial growth (Klebsiella pneumonia and Staphylococcus aureus) by the [RuIII(pic)3] complex both in absence and presence of cysteine has been examined, and the results show a positive effect of cysteine addition on the anti-bacterial activity of the complex for S. aureus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


