手指如何影响拇指的折叠:SARS-CoV-2 RdRp 蛋白折叠过程中的亚域间合作
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
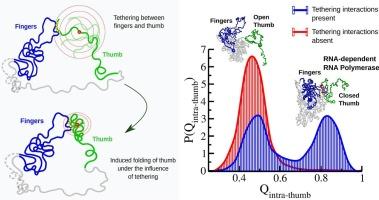
SARS-CoV-2 的 RNA 依赖性 RNA 聚合酶(RdRp)是病毒复制和转录所必需的关键酶,因此成为关键的治疗靶点。RdRp 蛋白具有独特的右手杯状结构,有两个重要的子域:手指和拇指。尽管各不相同,但生物物理实验表明,这两个亚域相互合作,促进 RNA 的容纳,确保了 RdRp 的功能。为了探究apo和RNA结合的RdRp中手指与拇指相互作用的结构机制,我们根据最近的冷冻电镜数据构建了一个粗粒度的结构模型。模拟结果表明,在 apo RdRp 中,从打开到关闭的构象转变非常频繁,类似于呼吸运动。这些构象变化受手指与拇指的关联以及拇指亚域的折叠动力学的调控。拇指只有在被手指-拇指界面拴住时才会采用稳定的折叠;当这些子域断开时,拇指就会过渡到开放状态。通过分析大量从打开到关闭的转换事件,生成了转换接触概率图,该图突出显示了拇指-手指界面上远离 RNA 容纳位点的几个特定残基,它们对于诱导拇指的折叠过程至关重要。鉴于拇指亚域的折叠对 RNA 结合和病毒复制至关重要,该研究认为这些界面残基可能具有远程调控开关的功能,可以作为开发针对 SARS-CoV-2 和类似 RNA 病毒的异构药物的目标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
How fingers affect folding of a thumb: Inter-subdomain cooperation in the folding of SARS-CoV-2 RdRp protein
The RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 is a critical enzyme essential for the virus's replication and transcription, making it a key therapeutic target. The RdRp protein exhibits a characteristic cupped right-hand shaped structure with two vital subdomains: the fingers and the thumb. Despite being distinct, biophysical experiments suggest that these subdomains cooperate to facilitate RNA accommodation, ensuring RdRp functionality. To investigate the structure-based mechanisms underlying the fingers-thumb interaction in both apo and RNA-bound RdRp, we constructed a coarse-grained structure-based model based on recent cryo-electron microscopy data. The simulations reveal frequent open-to-closed conformational transitions in apo RdRp, akin to a breathing-like motion. These conformational changes are regulated by the fingers-thumb association and the folding dynamics of the thumb subdomain. The thumb adopts a stable fold only when tethered by the fingers-thumb interface; when these subdomains are disconnected, the thumb transitions into an open state. A significant number of open-to-closed transition events were analyzed to generate a transition contact probability map, which highlights a few specific residues at the thumb-fingers interface, distant from the RNA accommodation sites, as essential for inducing the thumb's folding process. Given that thumb subdomain folding is critical for RNA binding and viral replication, the study proposes that these interfacial residues may function as remote regulatory switches and could be targeted for the development of allosteric drugs against SARS-CoV-2 and similar RNA viruses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


