海洋羟基磷灰石的特性及其在兔子股骨缺损模型中的骨填充作用:体内、体外和硅学研究
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
目的羟基磷灰石(HA)是一种用于骨缺损治疗的前景广阔的支架材料。本研究旨在评估一种从蓝鱼鳞片中提取的新型海洋来源 HA 的物理化学和生物学特性,并评估其作为骨替代物用于临床的潜力。方法将 80 只雌性新西兰白兔分为 4 组(n = 20):HA 粉(EC 组)、3D 打印 HA 柱(I3DFFF 组)、阴性对照组(NC 组;无生物材料的骨缺损)和阳性对照组(PC 组;用商业合成的 HA 治疗骨缺损)。生物材料的体外表征采用了 X 射线衍射、氮吸附、扫描电子显微镜、发射分光光度法和 MTT 分析法。体内评估也采用了相同的技术,并在植入后 1、3、6 和 9 个月进行了组织学和放射学分析。此外,还进行了分子对接研究,以调查 HA 对 RANKL 诱导的破骨细胞生成和 NF-κB 信号通路的影响。结果体外表征显示,海洋提取的 HA 是一种介孔材料,其晶体结构与天然骨相似。在体内,该材料表现出生物相容性、骨诱导性和骨传导性,为骨再生提供了支持性环境。分子对接表明,HA 可通过抑制 NF-κB 信号通路来抑制破骨细胞的生成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
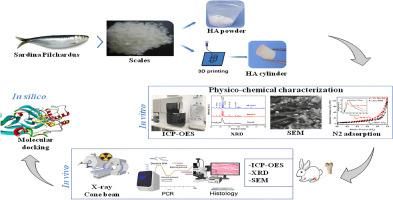
Characterization of marine hydroxyapatite and its interest in bone filling in rabbits femoral defect model: In vivo, in vitro, and in silico study
Purpose
Hydroxyapatite (HA) is a promising scaffold material for bone defect treatment. This study aimed to evaluate the physicochemical and biological properties of a novel marine-derived HA extracted from blue fish scales and assess its potential as a bone substitute for clinical use.
Methods
80 female New Zealand white rabbits were assigned to 4 groups (n = 20): HA powder (EC group), 3D-printed HA cylinder (I3DFFF group), negative control group (NC group; bone defect without biomaterial), and positive control group (PC group; bone defect treated with commercially synthesized HA). The biomaterials were characterized in vitro using X-ray diffraction, nitrogen adsorption, scanning electron microscopy, emission spectrophotometry, and MTT assays. In vivo evaluation involved the same techniques, supplemented with histological and radiological analyses at 1, 3, 6, and 9 months post-implantation. Molecular docking studies were also performed to investigate HA's effect on RANKL-induced osteoclastogenesis and the NF-κB signaling pathway.
Results
In vitro characterization showed that marine-derived HA is a mesoporous material with a crystalline structure similar to natural bone. In vivo, the material demonstrated biocompatibility, osteoinductivity, and osteoconductivity, providing a supportive environment for bone regeneration. Molecular docking indicated that HA could inhibit osteoclastogenesis by suppressing the NF-κB signaling pathway.
Conclusion
Our findings suggest that the two co-products, EC-17 and I3DFFF, possess the necessary physicochemical and biological characteristics to be developed as effective bone substitutes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


