固态合成用于高性能超级电容器的硒化镍
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
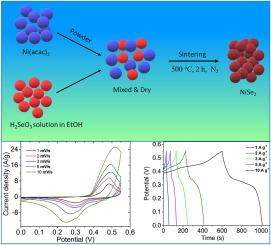
本研究的重点是二硒化镍(NiSe2)的合成和电化学特性,它是一种很有前途的超级电容器电极材料。NiSe2 是通过一种简便的固态工艺合成的,该工艺涉及乙酰丙酮镍和亚硒酸的混合,然后在 500 °C 的惰性条件下进行干燥和烧结。得到的 NiSe2 呈颗粒状结构,表面结构呈蠕虫状,粒径在 20 纳米到 100 纳米之间。在 6 M KOH 电解液中,使用循环伏安法(CV)、电静态充放电法(GCD)和电化学阻抗谱法(EIS)对 NiSe2 的电化学性能进行了评估。NiSe2 在放电速率为 1 A g-1 时的比电容高达 744.7 F g-1,在 10 A g-1 时的比电容保持率为 483.6 F g-1,并且具有出色的长期循环稳定性。动力学分析表明,NiSe2 的储能机制主要涉及扩散控制的电荷存储。EIS 进一步证实了 NiSe2 电极良好的电荷转移特性。总之,通过所提出的方法合成的 NiSe2 在高性能超级电容器中的应用前景广阔。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Solid-state synthesis of nickel selenide for high-performance supercapacitors
This study focuses on the synthesis and electrochemical characterization of nickel diselenide (NiSe2) as a promising electrode material for supercapacitors. NiSe2 was synthesized through a facile solid-state process involving the mixing of nickel acetylacetonate and selenous acid, followed by drying and sintering at 500 °C under inert conditions. The resulting NiSe2 exhibited a granular structure with worm-like surface architecture and particle size ranging from 20 to 100 nm. The electrochemical performance of NiSe2 was evaluated using cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS) in a 6 M KOH electrolyte. NiSe2 demonstrated a high specific capacitance of 744.7 F g−1 at a discharge rate of 1 A g−1, with an outstanding rate capability retaining the capacitance of 483.6 F g−1 at 10 A g−1, and exceptional long-term cycling stability. The kinetic analysis revealed that the energy storage mechanism in NiSe2 primarily involves diffusion-controlled charge storage. EIS further confirmed the favorable charge transfer properties of the NiSe2 electrode. Overall, NiSe2 synthesized via the proposed method shows great promise for application in high-performance supercapacitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


