开发和验证用于非法药物和辅料完整特征分析的法医工作流程
IF 2.6
3区 医学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
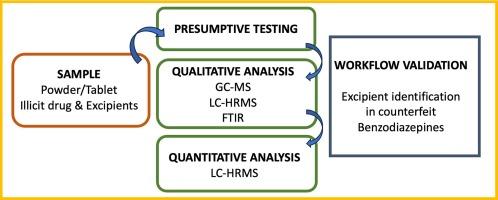
要全面了解非法药物混合物的社会危害,就必须更加重视非法化合物和辅料化合物的全面鉴定。为了提高这种做法在实践中的可行性,在开发非目标法医学工作流程的背景下,对常见的和新兴的分析技术进行了研究。该工作流程的目的是在不影响非法药物鉴定质量的情况下,提高辅料化合物的鉴定率,这也是法庭采纳证据的要求。其中包括对模拟化合物混合物进行测试,以开发主要的分析途径。然后通过测试未知化合物混合物来验证这些途径。重点技术包括 GCMS、FTIR、用于鉴定的 LC-HRMS 和用于定量的 LC-HRMS。根据 SWGDRUG 指南,这些技术被归入各自的技术类别,以形成一个工作流程,确保无论采用哪种途径,证据都具有可采性。HRMS 作为一种目前尚未用于非法药物分析的新兴技术,为非目标分析途径提供了便利。由此,结合 GCMS,可以识别模拟和未知混合物中的所有有机成分。利用傅立叶变换红外分析法还对不溶性化合物进行了部分鉴定。通过与参考标准的比较以及与高分辨率数据库 MzCloud 的 MS/MS 光谱匹配,促进了 HRMS 的鉴定。这表明 HRMS(特别是 Exploris 120 Orbitrap)适用于法医化学中非法和有机辅料化合物的鉴定和定量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development and validation of a forensic workflow for the complete profiling of illicit drugs and excipients
The development of a complete understanding of the societal harms of illicit drug mixtures requires a greater emphasis on the complete identification of illicit and excipient compounds. To increase the feasibility of this in practice, common and emerging analytical techniques were examined in the context of developing a non-targeted forensic workflow. The purpose of this workflow was to increase the identification of excipient compounds without compromising the quality of illicit drug identification as required for admissibility of evidence in court. This incorporated the testing of simulated compound mixtures to develop the principal avenues of analysis. These pathways were then validated through the testing of unknown compound mixtures. The techniques of focus included GCMS, FTIR, LC-HRMS for identification, and LC-HRMS for quantitation. These techniques were organised into their respective categories of techniques, according to the SWGDRUG guidelines, to produce a workflow that would ensure the admissibility of evidence no matter the pathway taken. HRMS was examined as an emerging technique not currently used in illicit drug analysis facilitating non-targeted analysis pathways. From this, and in combination with GCMS, all organic components were identifiable in simulated and unknown mixtures. Partial identification was also achieved for insoluble compounds using FTIR analysis. Identification by HRMS was facilitated by comparison to reference standards and MS/MS spectra matching to the high-resolution database MzCloud. This demonstrated the applicability of HRMS, specifically the Exploris 120 Orbitrap, to the identification and quantitation of both illicit and organic excipient compounds within Forensic Chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Forensic Chemistry
CHEMISTRY, ANALYTICAL-
CiteScore
5.70
自引率
14.80%
发文量
65
审稿时长
46 days
期刊介绍:
Forensic Chemistry publishes high quality manuscripts focusing on the theory, research and application of any chemical science to forensic analysis. The scope of the journal includes fundamental advancements that result in a better understanding of the evidentiary significance derived from the physical and chemical analysis of materials. The scope of Forensic Chemistry will also include the application and or development of any molecular and atomic spectrochemical technique, electrochemical techniques, sensors, surface characterization techniques, mass spectrometry, nuclear magnetic resonance, chemometrics and statistics, and separation sciences (e.g. chromatography) that provide insight into the forensic analysis of materials. Evidential topics of interest to the journal include, but are not limited to, fingerprint analysis, drug analysis, ignitable liquid residue analysis, explosives detection and analysis, the characterization and comparison of trace evidence (glass, fibers, paints and polymers, tapes, soils and other materials), ink and paper analysis, gunshot residue analysis, synthetic pathways for drugs, toxicology and the analysis and chemistry associated with the components of fingermarks. The journal is particularly interested in receiving manuscripts that report advances in the forensic interpretation of chemical evidence. Technology Readiness Level: When submitting an article to Forensic Chemistry, all authors will be asked to self-assign a Technology Readiness Level (TRL) to their article. The purpose of the TRL system is to help readers understand the level of maturity of an idea or method, to help track the evolution of readiness of a given technique or method, and to help filter published articles by the expected ease of implementation in an operation setting within a crime lab.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


