通过密度泛函理论计算对 B 位掺杂 Pr(Ba,Sr)Co2O5+δ的氧还原机制进行原子尺度的机理研究
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
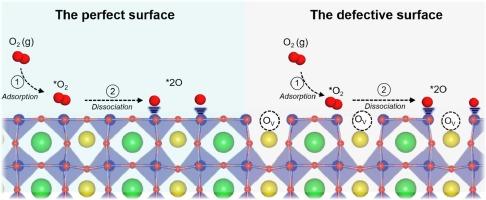
Pr(Ba,Sr)Co2O5+δ具有很高的氧离子传输能力和氧还原活性,是一种很有前途的固体氧化物燃料电池(SOFC)阴极材料。为了阐明 B 位掺杂 Pr(Ba,Sr)(Co,M)2O5+δ(M = Fe、Ni、Cu 和 Zn)材料的氧还原机制,我们进行了密度泛函理论(DFT)计算。首先,我们研究了 O 空位的形成。结果表明,Cu 和 Zn 的掺杂促进了 O 空位的形成,从而降低了 O 空位的形成能。此外,我们还全面讨论了氧与 B 位掺杂的 Pr(Ba,Sr)(Co,M)2O5+δ(0 0 1)表面之间的相互作用,包括完美表面和缺陷表面。结果表明,O 空位的存在可降低 O2 解离所需的能量,从而增强氧还原的催化活性。掺锌的 Pr(Ba,Sr)(Co,M)2O5+δ具有较低的 O 空位形成能,从而在氧气解离时形成稳定的吸附构型,这表明它具有作为 SOFC 阴极材料的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Atomic-scale mechanistic study of oxygen reduction mechanism for B-site doped Pr(Ba,Sr)Co2O5+δ by density functional theory calculations
Pr(Ba,Sr)Co2O5+δ is a promising cathode material for solid oxide fuel cell (SOFC) due to its high oxygen ion transport capability and oxygen reduction activity. Density functional theory (DFT) calculations were performed to elucidate the oxygen reduction mechanism of B-site doped Pr(Ba,Sr)(Co,M)2O5+δ (M = Fe, Ni, Cu, and Zn) materials. First, we investigated the formation of O vacancies. The results indicate that Cu and Zn doping facilitate O vacancy formation, resulting in lower O vacancy formation energies. Furthermore, the interaction between oxygen and the (0 0 1) surfaces of B-site doped Pr(Ba,Sr)(Co,M)2O5+δ has been comprehensively discussed, involving both perfect and defective surfaces. The results demonstrate that the presence of O vacancies enhances the catalytic activity for oxygen reduction, by reducing the energy required for O2 dissociation. Zn-doped Pr(Ba,Sr)(Co,M)2O5+δ exhibits a low O vacancy formation energy, resulting in a stable adsorption configuration upon oxygen dissociation, indicating its potential as a cathode material for SOFC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


