关于 BaO(BaCO3)-Lu2O3-CuO 体系的更多信息
IF 3.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在 900 °C 下绘制了 BaO(BaCO3)-Lu2O3-CuO 体系的相图。它包括 8 个单相区域、15 个两相区域和 8 个三相区域。边界氧化物体系 Lu2O3-CuO 和 BaO-CuO 包含相 Lu2Cu2O5(结构类型 Ho2Cu2O5,皮尔逊符号 oP36,空间群 Pna21,a = 10.702(1),b = 3.412(1),c = 12.360(1) Å,RB = 0.076)和 Ba44Cu45O90(自身结构类型,cI400,Im-3m,a = 18.294(3)埃,RB = 0.114),而在 Lu2O3-BaO(BaCO3)体系中,在实验条件下发现了氧化物-碳酸盐 Ba3Lu2[CO3]O5 (Ba3Yb2[CO3]O5, tP26, P4/mmm, a = 4.322(1), c = 11.862(2) 埃,RB = 0.107)。观察到两种四氧化物。BaLu2CuO5 的结构已得到确认(BaY2CuO5, oP36, Pnma),而近似成分为 Ba3LuCu2O6.5 的相的结构仍有待确定。研究了锂离子电池中作为阴极材料的 BaLu2CuO5 的电化学特性。单位晶胞体积的减小(-0.11 %)证实了(部分)置换固溶体 BaLu2CuO5:Li 的存在。在 900 °C 温度下,没有检测到形成 YBCO 型结构相的痕迹。本文章由计算机程序翻译,如有差异,请以英文原文为准。
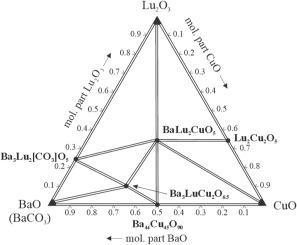
More about the BaO(BaCO3)–Lu2O3–CuO system
The phase diagram of the BaO(BaCO3)–Lu2O3–CuO system was built at 900 °C. It comprises of 8 single-phase, 15 two-phase, and 8 three-phase regions. The boundary oxide systems Lu2O3–CuO and BaO–CuO contain the phases Lu2Cu2O5 (structure type Ho2Cu2O5, Pearson symbol oP36, space group Pna21, a = 10.702(1), b = 3.412(1), c = 12.360(1) Å, RB = 0.076) and Ba44Cu45O90 (own structure type, cI400, Im-3m, a = 18.294(3) Å, RB = 0.114), whereas in the Lu2O3–BaO(BaCO3) system the oxide-carbonate Ba3Lu2[CO3]O5 (Ba3Yb2[CO3]O5, tP26, P4/mmm, a = 4.322(1), c = 11.862(2) Å, RB = 0.107) was identified under the conditions of the experiment. Two quaternary oxides were observed. The structure of BaLu2CuO5 was confirmed (BaY2CuO5, oP36, Pnma), whereas the structure of the phase with approximate composition Ba3LuCu2O6.5 still needs to be established. The electrochemical properties of BaLu2CuO5 as cathode material in Li-ion batteries were investigated. The existence of a (in part) substitutional solid solution BaLu2CuO5:Li was confirmed by the decrease of the unit-cell volume (−0.11 %). No traces of the formation of a phase with YBCO-type structure were detected at 900 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


