狼疮小鼠体内的 B 细胞内源性 IFN-γ 促进了 CD11c+ 年龄相关 B 细胞的过度分化,并损害了生殖中心的选择能力
IF 2.9
4区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
CD11c+ 年龄相关 B 细胞(ABC)已成为保护性和自反应性 B 细胞反应的关键组成部分。红斑狼疮是一种自身免疫性疾病,与疫苗效力降低和感染易感性增加有关。以前,我们曾报道过,过多的 CD11c+ ABCs 不仅能显著促进自身抗体的产生,还能促进狼疮小鼠异常的 T 细胞活化,并影响其对免疫的亲和性生殖中心选择。然而,CD11c+ ABC分化的调控机制尚未完全明了。本研究表明,狼疮小鼠 CD11c+ ABC 过度分化需要 B 细胞内源性 IFN-γ。B 细胞内源性 IFN-γ 主要由 CD11c+ ABCs 产生。IFN-γ缺乏会导致ABC特征基因的表达减少。我们进一步发现,消减 IFN-γ 可使狼疮小鼠的 T 细胞过度激活恢复正常,并挽救抗原特异性 GC 反应。我们的研究深入揭示了B细胞内源性IFN-γ在促进CD11c+ ABC过度分化中的关键作用,它损害了狼疮中基于亲和力的生殖中心选择和亲和力成熟,为狼疮疫苗反应正常化提供了一种潜在的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
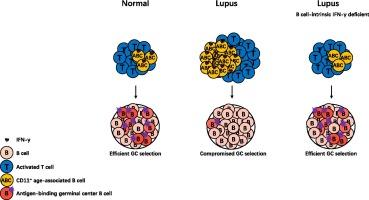
B cell-intrinsic IFN-γ promotes excessive CD11c+ age-associated B cell differentiation and compromised germinal center selection in lupus mice
CD11c+ age-associated B cells (ABCs) have emerged as a key component in protective and autoreactive B cell responses. Lupus is an autoimmune disorder linked to reduced efficacy of vaccines and increased susceptibility to infections. Previously, we reported that excessive CD11c+ ABCs not only significantly contribute to autoantibody production but also promote aberrant T cell activation and compromised affinity-based germinal center selection in response to immunization in lupus mice. Yet, the regulation of CD11c+ ABC differentiation is not fully understood. In this study, we show that B cell-intrinsic IFN-γ is required for excessive CD11c+ ABC differentiation in lupus mice. B cell-intrinsic IFN-γ is mainly produced by CD11c+ ABCs. IFN-γ-deficiency leads to decreased expression of ABC characteristic genes. We further show that ablating IFN-γ can normalize T cell overactivation and rescue antigen-specific GC responses in lupus mice. Our study offers insight into the crucial role of B cell-intrinsic IFN-γ in promoting excessive CD11c+ ABC differentiation, which compromises affinity-based germinal center selection and affinity maturation in lupus, providing a potential strategy to normalize vaccine responses in lupus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular immunology
生物-免疫学
CiteScore
8.20
自引率
2.30%
发文量
102
审稿时长
30 days
期刊介绍:
Cellular Immunology publishes original investigations concerned with the immunological activities of cells in experimental or clinical situations. The scope of the journal encompasses the broad area of in vitro and in vivo studies of cellular immune responses. Purely clinical descriptive studies are not considered.
Research Areas include:
• Antigen receptor sites
• Autoimmunity
• Delayed-type hypersensitivity or cellular immunity
• Immunologic deficiency states and their reconstitution
• Immunologic surveillance and tumor immunity
• Immunomodulation
• Immunotherapy
• Lymphokines and cytokines
• Nonantibody immunity
• Parasite immunology
• Resistance to intracellular microbial and viral infection
• Thymus and lymphocyte immunobiology
• Transplantation immunology
• Tumor immunity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


