含有源自手性氨基醇的膦配体的高级设计 Ru(II) 复合物:电化学行为、DFT 计算和生物活性
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
我们报告了两种来自手性氨基醇的膦配体及其与钌(II)的配合物。这些化合物通过光谱方法进行了表征。然后,测试了手性 Ru(II)-phosphinite 复合物的抗菌、抗氧化和 DNA 结合活性。(1R)-2-{苄基[(1S)-1-(萘-1-基)乙基]氨基}-1-苯乙基二苯基磷[二氯(η6-对伞花烃)钌(II)]络合物 7 在 200.0 mg l-1 浓度下表现出最高的自由基清除活性(90.93 ± 0.98 %)和最高的金属螯合活性(65.45 ± 1.46 %)。此外,所有复合物与小牛胸腺 DNA(CT-DNA)的结合率也各不相同。此外,研究人员还进行了广泛的理论和实验研究,以更深入地了解钌配合物的化学描述和发生的各种电子转变,以及它们的电化学特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
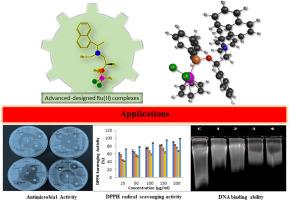
Advanced-designed Ru(II) complexes containing phosphinite ligands derived from chiral amino alcohols: Electrochemical behavior, DFT calculations, and biological activity
We report two phosphinite ligands derived from chiral amino alcohols, and their complexes with ruthenium(II). These compounds were characterized by spectroscopic methods. Then, antimicrobial, antioxidant, and DNA binding activities of the chiral Ru(II)-phosphinite complexes were tested. Complex (1R)-2-{benzyl[(1S)-1-(naphthalen-1-yl)ethyl]amino}-1-phenylethyl diphenylphos- phinito[dichloro(η6-p-cymene)ruthenium(II)], 7 showed both the highest radical scavenging activity (90.93 ± 0.98 %) and the highest metal chelating activity (65.45 ± 1.46 %) at 200.0 mg l-1 concentration. In addition, all of complexes had different rates of binding activity to calf thymus DNA (CT-DNA). Moreover, extensive theoretical and experimental investigations were conducted to gain a more profound understanding of the chemical descriptors and the diverse electronic transitions taking place within the ruthenium complexes, as well as their electrochemical characteristics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


