镓锗二元系统的严格评估和热力学 CALPHAD 重新评估
IF 0.7
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在 CALPHAD(CALculation of PHAse Diagram)方法的帮助下,对镓-锗二元系统进行了热力学建模。液相和金刚石(Ge)相用亚晶格形式主义描述,过剩模型用 Redlich-Kister 方程描述。溶液相(Ga)被视为纯元素。从文献中收集到的大部分实验数据,包括相图和热力学信息,都被用来优化热力学参数。计算得出的相图和系统的热力学性质与大多数实验数据相符。本文章由计算机程序翻译,如有差异,请以英文原文为准。
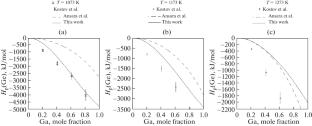
Critical Evaluation and Thermodynamic CALPHAD Reassessment of the Gallium–Germanium Binary System
Thermodynamic modeling of the Ga–Ge binary system was carried out with the help of the CALPHAD (CALculation of PHAse Diagram) method. The liquid and diamond (Ge) phases have been described with the sublattice formalism and the excess model with the Redlich–Kister equation. The solution phase (Ga) was treated as a pure element. The most experimental data, including phase diagrams and thermodynamic information, gathered from the literature were employed to optimize the thermodynamic parameters. The resultant calculated phase diagram and the system’s thermodynamic properties are in satisfactory accordance with the majority of the experimental data.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
14.30%
发文量
376
审稿时长
5.1 months
期刊介绍:
Russian Journal of Physical Chemistry A. Focus on Chemistry (Zhurnal Fizicheskoi Khimii), founded in 1930, offers a comprehensive review of theoretical and experimental research from the Russian Academy of Sciences, leading research and academic centers from Russia and from all over the world.
Articles are devoted to chemical thermodynamics and thermochemistry, biophysical chemistry, photochemistry and magnetochemistry, materials structure, quantum chemistry, physical chemistry of nanomaterials and solutions, surface phenomena and adsorption, and methods and techniques of physicochemical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


