对多孔钼酸铋进行改性,以提高抗生素去除率和 H2O2 产率†。
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Bi2MoO6 结构的调节,如掺杂剂的加入、复合材料的形成和合成条件的改变,因其对提高光催化活性的影响而备受关注。本研究通过一锅水热法在合成 Bi2MoO6 时引入了醋酸钾,以提高其光催化效率。研究发现,醋酸钾的加入有效地改变了 Bi2MoO6 的微观结构。XRD 和 XPS 分析证实,K+ 离子进入了 [MoO4]2- 和 [Bi2O2]2+ 层,显著影响了 Bi2MoO6 的相结构和形态。控制醋酸钾的添加量可改善 Bi2MoO6 的分散性,而过量添加则会导致 Bi2MoO6 向 Bi3.64Mo0.36O6.55 的相变。抗生素降解率和 H2O2 产率被用来评估催化剂的催化性能。与未改性的 Bi2MoO6 相比,用醋酸钾改性的 Bi2MoO6 表现出更高的光催化效率。具体来说,最佳的 BMO-1 样品在 15 分钟的光照时间内可实现 97.7% 的 CIP 降解。吸附效率的提高主要归功于有效分散和介孔的存在。此外,氧空位的引入和光生载流子分离效率的提高也有助于增强光催化性能。本研究介绍了一种从结构上调整钼酸铋的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
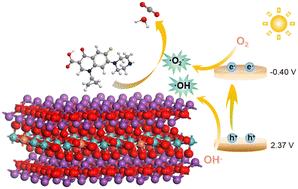
Modification of porous bismuth molybdate for high removal of antibiotics and H2O2 production†
The regulations of the Bi2MoO6 structure, such as dopant incorporation, composite formation, and synthesis condition modification, have garnered significant attention due to their implications for enhancing photocatalytic activity. In this study, potassium acetate was introduced into the synthesis of Bi2MoO6via a one-pot hydrothermal method to augment its photocatalytic efficiency. It was observed that the addition of potassium acetate effectively modulated the microstructure of Bi2MoO6. XRD and XPS analyses confirmed the incorporation of K+ ions into the [MoO4]2− and [Bi2O2]2+ layers, significantly influencing the phase structure and morphology of Bi2MoO6. Controlled addition of potassium acetate improved the dispersibility of Bi2MoO6, whereas excessive amounts led to a phase transition from Bi2MoO6 to Bi3.64Mo0.36O6.55. The antibiotic degradation rate and H2O2 yield were used to evaluate the catalytic performance of the catalyst. Bi2MoO6 modified with potassium acetate exhibited higher photocatalytic efficiency than unmodified Bi2MoO6. Specifically, the optimal BMO-1 sample exhibited 97.7% CIP degradation within 15 min of illumination. The enhanced adsorption efficiency was primarily attributed to the effective dispersion and the presence of mesopores. Furthermore, the introduction of oxygen vacancies and improved photogenerated carrier separation efficiency contributed to enhanced photocatalytic performance. This study introduces a novel method for structurally tuning bismuth molybdate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


