异质结构工程构建掺杂 N 的 CuO@Co3O4 用于高效电催化硝酸盐还原成氨†。
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在温和的条件下,电化学还原硝酸盐可以替代工业上使用的、高能耗的哈伯-博施工艺,是一种前景广阔的可持续工艺,同时还能解决水中硝态氮的污染问题。我们在此介绍在泡沫镍上简便构建掺杂 N 的 CuO@Co3O4/NF 纳米阵列,用于在中性电解质中通过选择性还原 NO3- 高效电合成 NH3。在-0.85 V电压下(相对于可逆氢电极(RHE)),当在添加了50 mM NaNO3的0.1 M磷酸盐缓冲溶液中操作时,这种N掺杂CuO@Co3O4/NF纳米阵列获得了99.78%的出色法拉第效率(FE)和高达31.92 mg h-1 cm-2的NH3产率。此外,根据表征结果,电催化性能的增强可归因于异质结构中 N 掺杂与相对大量的 Co(II) 和氧空位(OVs)的协同作用,从而抑制了氢进化反应(HER),改善了 N 掺杂 CuO@Co3O4/NF 异质结构的电子/质量传递。本文章由计算机程序翻译,如有差异,请以英文原文为准。
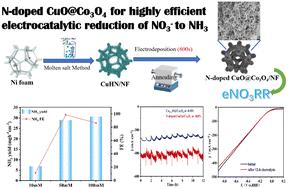
Heterostructure engineered construction of N-doped CuO@Co3O4 for highly efficient electrocatalytic reduction of nitrate to ammonia†
The electrochemical reduction of nitrate can be a promising and sustainable alternative for the industrially used, energy-intensive Haber–Bosch process under mild conditions and also solve the issue of nitrate nitrogen pollution in water. We present here the facile construction of an N-doped CuO@Co3O4/NF nanoarray on nickel foam for highly efficient NH3 electrosynthesis via selective NO3− reduction in neutral electrolyte. At −0.85 V (versus the reversible hydrogen electrode (RHE)), this N-doped CuO@Co3O4/NF nanoarray obtained an outstanding Faraday efficiency (FE) of 99.78% and NH3 yield of up to 31.92 mg h−1 cm−2 when operated in 0.1 M phosphate buffer solution with added 50 mM NaNO3. In addition, its excellent electrochemical performance was maintained for at least 12 h. Furthermore, according to the characterization, the enhanced electrocatalytic performance could be attributed to the synergistic effect of N-dopant and relatively large amounts of Co(ii) and oxygen vacancies (OVs) in the heterostructure, leading to the suppression of the hydrogen evolution reaction (HER) and the improvement of electron/mass transfer at the N-doped CuO@Co3O4/NF heterostructure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


