通过 Ylide 形成和 1,4-磺酰基重排对碳化铑进行氨磺酰化。
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在此报告了在基于氧-吡啶鎓酰亚胺化学的双官能化反应中使用吡啶-2-基苯磺酸盐作为磺化试剂的情况,为在一个分子中同时安装磺酰基和吡啶酮分子提供了有效的方案。密度泛函理论(DFT)计算显示,该反应过程可能是通过依次形成金属结合的醯胺、酮烯醇同分异构以及磺酰基的迁移重排进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
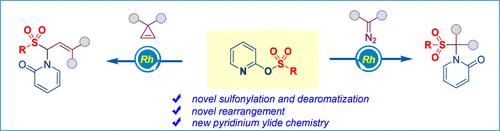
Aminosulfonylation of Rhodium Carbene via Ylide Formation and 1,4-Sulfonyl Rearrangement.
We report here the use of pyridin-2-yl benzenesulfonates as sulfonylation reagents in a difunctionalization reaction based on oxy-pyridinium ylide chemistry, providing an effective protocol for the installation of both a sulfonyl group and a pyridone moiety into one molecule. Density functional theory (DFT) calculations disclose that the reaction process might proceed through sequential metal-bound ylide formation, keto-enol tautomerism, and the migratory rearrangement of the sulfonyl group.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


