针对癌症患者的运动疗法数字化分散试验
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
我们开发并评估了运动数字平台(DPEx):这是一种分散式、以患者为中心的方法,旨在加强运动疗法临床研究的各个方面。DPEx 将提供跑步机与远程医疗和远程生物样本采集相结合,使所有研究程序都能在患者家中进行。通过链接健康生物设备,可以对生活方式和生理反应进行高分辨率监测。在此,我们介绍了 DPEx 的原理和开发过程,以及在三组不同癌症患者中进行的可行性评估:在三名治疗后原发性乳腺癌女性患者中进行的 0a 期开发研究;在 13 名未经治疗的实体瘤患者中进行的新辅助运动疗法 0b 期概念验证试验;以及在 53 名局部前列腺癌男性患者中进行的新辅助运动疗法 1a 期水平测定试验。总之,我们的研究证明了在临床人群中开展运动疗法临床试验的全数字化、分散式方法的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
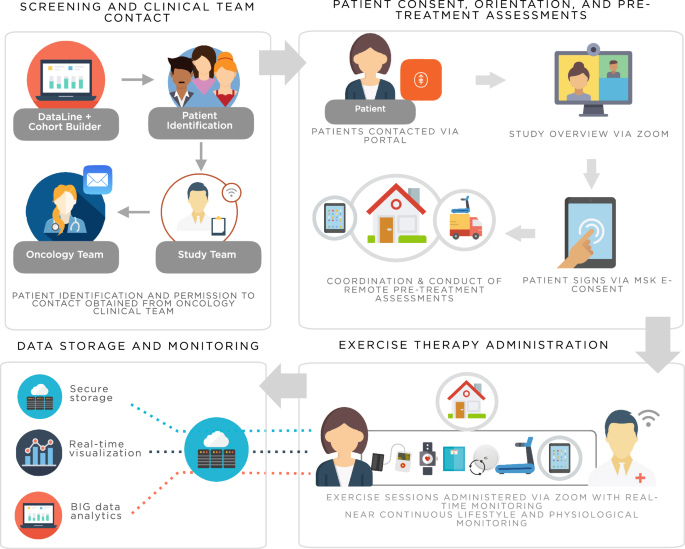
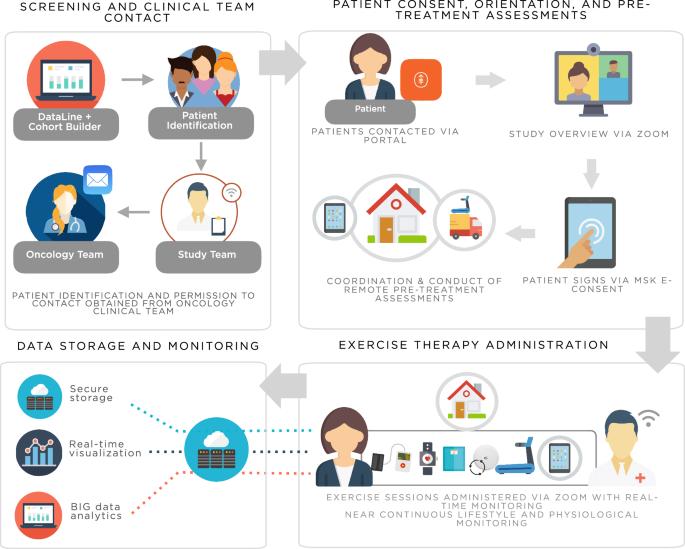
A digital, decentralized trial of exercise therapy in patients with cancer
We developed and evaluated the Digital Platform for Exercise (DPEx): a decentralized, patient-centric approach designed to enhance all aspects of clinical investigation of exercise therapy. DPEx integrated provision of a treadmill with telemedicine and remote biospecimen collection permitting all study procedures to be conducted in patient’s homes. Linked health biodevices enabled high-resolution monitoring of lifestyle and physiological response. Here we describe the rationale and development of DPEx as well as feasibility evaluation in three different cohorts of patients with cancer: a phase 0a development study among three women with post-treatment primary breast cancer; a phase 0b proof-of-concept trial of neoadjuvant exercise therapy in 13 patients with untreated solid tumors; and a phase 1a level-finding trial of neoadjuvant exercise therapy in 53 men with localized prostate cancer. Collectively, our study demonstrates the utility of a fully digital, decentralized approach to conduct clinical trials of exercise therapy in a clinical population.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


