利用含有磺酰胺或生物素分子的香豆素合成和研究选择性人碳酸酐酶 IX、XII 抑制剂。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
众所周知,碳酸酐酶同工酶(CAs)IX 和 XII 在多种实体瘤的发病和发展过程中起着重要作用。在这种情况下,针对上述同工酶的选择性 CA 抑制剂(CAIs)是开发癌症靶向药物的有效策略。为此,我们合成了基于芳基磺酰胺或生物素支架杂交的新型香豆素衍生物,并将其作为四种不同的人类碳酸酐酶同工酶(hCA I、II、IX 和 XII)的抑制剂进行了测试。香豆素-磺酰胺衍生物 27 以硫脲分子和三唑为连接体,对 hCA XII 的抑制活性最高,抑制常数(KI)为 7.5 nM,对 hCA I 具有很好的选择性。化合物 32 是对 hCA IX(KI = 6.3 nM)最有效的抑制剂,比本文用作参考化合物的药物乙酰唑胺 AAZ(KI = 25 nM)强 4 倍,对 hCA I 和 II 具有显著的选择性。香豆素-生物素衍生物 37-39 对靶上酶(hCA IX 和 XII)表现出突出的选择性,似乎是设计 CAIs 的可行线索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
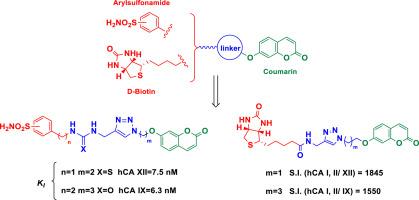
Synthesis and investigation of selective human carbonic anhydrase IX, XII inhibitors using coumarins bearing a sulfonamide or biotin moiety
The role of carbonic anhydrases isoforms (CAs) IX and XII in the pathogenesis and progression of many types of solid tumors is well known. In this context, selective CA inhibitors (CAIs) towards the mentioned isoforms is a validated strategy for the development of agents to target cancer. For this purpose, novel coumarin derivatives based on the hybridization with arylsulfonamide or biotin scaffolds were synthesized and tested as inhibitors of four different human carbonic anhydrases isoforms: hCA I, II, IX and XII. Coumarin-sulfonamide derived 27, with a thiourea moiety and triazole as linker, showed the highest inhibition activity against hCA XII with an inhibition constant (KI) of 7.5 nM and afforded a very good selectivity over hCA I. Compound 32 was the most potent inhibitor against hCA IX (KI = 6.3 nM), 4-fold stronger than the drug acetazolamide AAZ (KI = 25 nM), used herein as a reference compound, and showed remarkable selectivity over hCA I and II. The coumarin-biotin derivatives 37–39 showed outstanding selectivity towards on-target enzymes (hCA IX and XII) and appear as plausible leads for designing of CAIs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


