人类干细胞特异性表观遗传特征控制转基因表达。
IF 2.6
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et Biophysica Acta-Gene Regulatory Mechanisms
Pub Date : 2024-10-20
DOI:10.1016/j.bbagrm.2024.195063
引用次数: 0
摘要
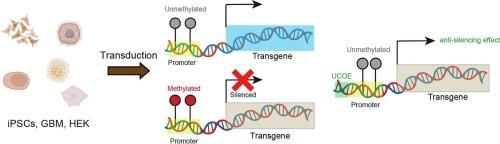
人类干细胞衍生模型已成为研究组织分化和疾病机制的重要平台。这些模型可以利用生化和细胞生物学方法,如omics、自噬和细胞器动力学。然而,干细胞中的表观遗传沉默为应用基因编码工具制造了障碍。在这里,我们通过在人类诱导多能干细胞(iPSC)、胶质母细胞瘤细胞(GBM)和胚胎肾细胞(HEK)中使用多种常用启动子,研究了外源表达基因沉默的分子机制。我们发现,与非 iPSCs 相比,所有测试的启动子在 CpG 岛区域的甲基化程度都很高,在 iPSCs 中的蛋白表达量较低。与其他启动子相比,伸长因子 1α 短(EF1α short 或 EFS)启动子的 CpG 岛数量较少,尽管存在 CpG 甲基化,但仍能在 iPSCs 中驱动相对较高的基因表达。在启动子上游添加最小A2泛在染色质开放元件(minimal A2 UCOE或miniUCOE)可抑制CpG甲基化,增强iPSCs中的基因表达。我们的研究结果证明了转基因启动子区域的干细胞特异性表观遗传修饰,并为设计抗沉默策略以提高转基因在iPSCs中的表达提供了有用的信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Human stem cell-specific epigenetic signatures control transgene expression
Human stem cell-derived models have emerged as an important platform to study tissue differentiation and disease mechanisms. Those models could capitalize on biochemical and cell biological methodologies such as omics, autophagy, and organelle dynamics. However, epigenetic silencing in stem cells creates a barrier to apply genetically encoded tools. Here we investigate the molecular mechanisms underlying exogenously expressed gene silencing by employing multiple commonly used promoters in human induced pluripotent stem cells (iPSCs), glioblastoma cells (GBM), and embryonic kidney cells (HEK). We discover that all promoters tested are highly methylated on the CpG island regions with lower protein expression in iPSCs, as compared to non-iPSCs. Elongation factor 1 alpha short (EF1α short or EFS) promoter, which has fewer CpG island number compared to the other promoters, can drive relatively higher gene expression in iPSCs, despite CpG methylation. Adding a minimal A2 ubiquitous chromatin opening element (minimal A2 UCOE or miniUCOE) upstream of a promoter inhibits CpG methylation and enhances gene expression in iPSCs. Our results demonstrate stem cell type-specific epigenetic modification of transgenic promoter region and provide useful information for designing anti-silencing strategies to increase transgene expression in iPSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.20
自引率
2.10%
发文量
63
审稿时长
44 days
期刊介绍:
BBA Gene Regulatory Mechanisms includes reports that describe novel insights into mechanisms of transcriptional, post-transcriptional and translational gene regulation. Special emphasis is placed on papers that identify epigenetic mechanisms of gene regulation, including chromatin, modification, and remodeling. This section also encompasses mechanistic studies of regulatory proteins and protein complexes; regulatory or mechanistic aspects of RNA processing; regulation of expression by small RNAs; genomic analysis of gene expression patterns; and modeling of gene regulatory pathways. Papers describing gene promoters, enhancers, silencers or other regulatory DNA regions must incorporate significant functions studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


