胃泌素细胞中的 Piezo1 机械传感有助于小鼠肝脏的脂质平衡。
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
胃泌素是胃泌素细胞释放的一种促食欲肽,是肥胖和肝脂肪变性的诱因。胃泌素细胞中的机械敏感性离子通道 Piezo1 在胃机械拉伸时抑制胃泌素的合成和分泌。我们试图通过操纵胃泌素细胞中的 Piezo1 来调节肝脏脂质代谢。胃泌素细胞特异性缺乏 Piezo1 的小鼠(Ghrl-Piezo1-/-)在摄入低脂或高脂饮食时会出现高胃泌素血症和肝脂肪变性。在这些小鼠中,肝脏脂质积累与基因表达、蛋白质丰度和活性的变化有关,这些变化预计会增加肝脏脂肪酸的合成并减少脂质的β-氧化。对胃泌素受体的药理抑制改善了 Ghrl-Piezo1-/- 小鼠的肝脏脂肪变性,从而证实了这些小鼠的表型是由于 Piezo1 失活导致胃泌素过度分泌造成的。在野生型小鼠胃中植入硅胶珠以诱导胃的机械性伸展,可抑制胃泌素的合成和分泌,从而有助于抑制高脂饮食诱导的脂肪肝的发生,而在Ghrl-Piezo1-/-小鼠中则没有这种作用。我们的研究阐明了胃胃泌素细胞中的Piezo1调节肝脏脂质积累的机制,为潜在的脂肪肝治疗提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
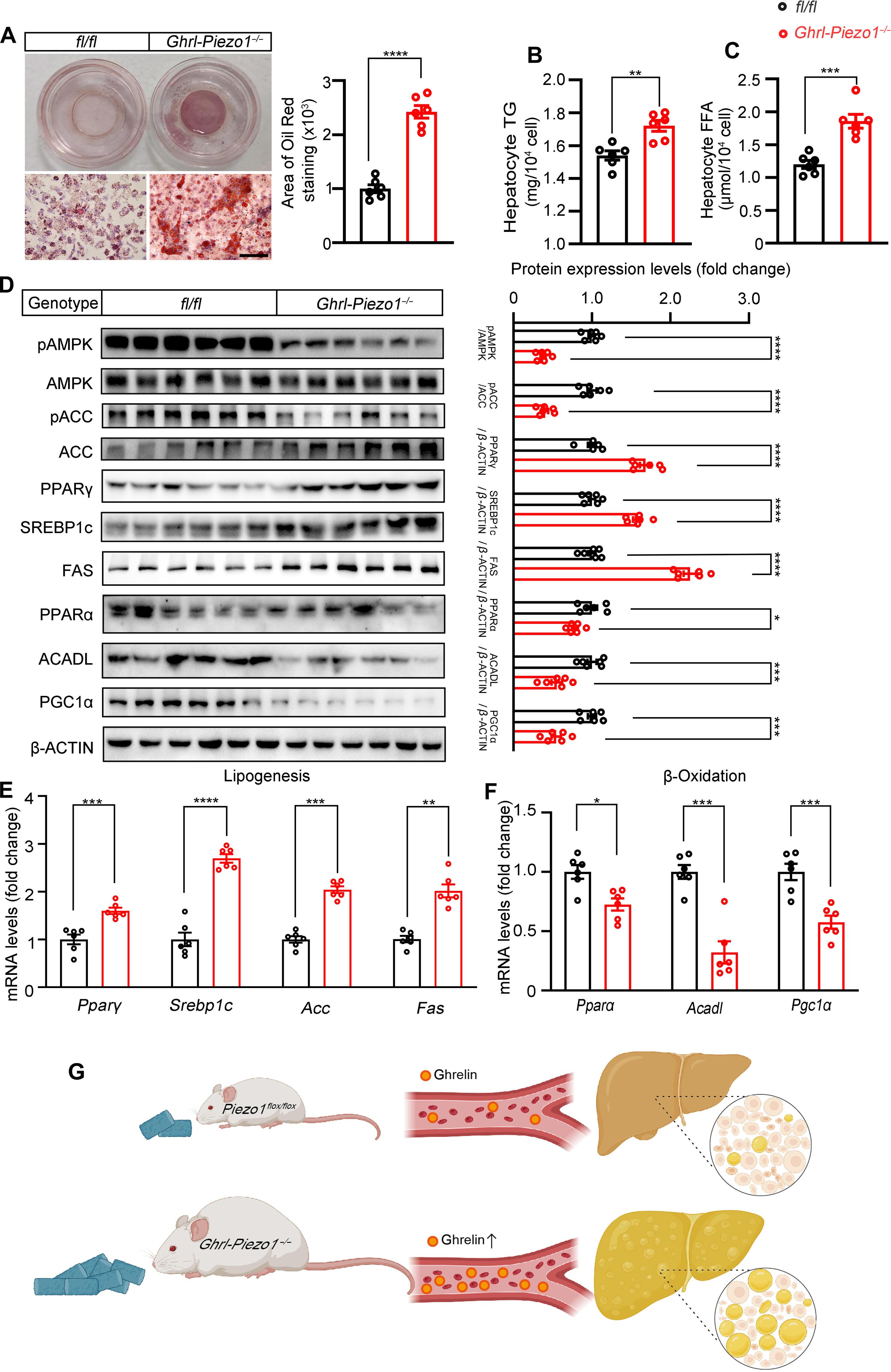
Mechanosensing by Piezo1 in gastric ghrelin cells contributes to hepatic lipid homeostasis in mice
Ghrelin is an orexigenic peptide released by gastric ghrelin cells that contributes to obesity and hepatic steatosis. The mechanosensitive ion channel Piezo1 in gastric ghrelin cells inhibits the synthesis and secretion of ghrelin in response to gastric mechanical stretch. We sought to modulate hepatic lipid metabolism by manipulating Piezo1 in gastric ghrelin cells. Mice with a ghrelin cell–specific deficiency of Piezo1 (Ghrl-Piezo1−/−) had hyperghrelinemia and hepatic steatosis when fed a low-fat or high-fat diet. In these mice, hepatic lipid accumulation was associated with changes in gene expression and in protein abundance and activity expected to increase hepatic fatty acid synthesis and decrease lipid β-oxidation. Pharmacological inhibition of the ghrelin receptor improved hepatic steatosis in Ghrl-Piezo1−/− mice, thus confirming that the phenotype of these mice was due to overproduction of ghrelin caused by inactivation of Piezo1. Gastric implantation of silicone beads to induce mechanical stretch of the stomach inhibited ghrelin synthesis and secretion, thereby helping to suppress fatty liver development induced by a high-fat diet in wild-type mice but not in Ghrl-Piezo1−/− mice. Our study elucidates the mechanism by which Piezo1 in gastric ghrelin cells regulate hepatic lipid accumulation, providing insights into potential treatments for fatty liver.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


