研究氧化应激的有前途的工具:非啮齿动物模式生物综述。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
氧化应激是氧化还原生物学中的一个重要概念,近年来已取得了重大进展。过量的活性氧(ROS)会导致氧化损伤,增加罹患各种疾病的风险。与此相反,ROS 保持在适度范围内对正常生理代谢起着调节作用。在复杂的研究环境中选择合适的动物模型对于提高研究效率至关重要。虽然啮齿类动物经常被用于医学实验,但它们也面临着高成本和伦理考虑等挑战。另外,斑马鱼、果蝇和优雅子等非啮齿类动物模型为氧化应激研究提供了很好的途径。这些生物体具有体积小、繁殖率高、可进行活体成像和易于基因操作等优点。本综述将重点介绍利用非啮齿类动物模型检测氧化应激的进展。本综述系统地综述了氧化平衡调控途径--Kelch样ECH相关蛋白1-核因子红细胞2相关因子2(Keap1-Nrf2),以及不同生物体中以Nrf2为中心的多种调控途径。最后,本综述对不同模式生物进行了全面的比较分析,并进一步探讨了新技术与非啮齿类动物的结合。本综述旨在总结利用非啮齿类动物进行氧化应激研究的最新发现,并划定未来的研究方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
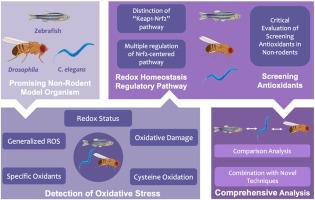
Promising tools into oxidative stress: A review of non-rodent model organisms
Oxidative stress is a crucial concept in redox biology, and significant progress has been made in recent years. Excessive levels of reactive oxygen species (ROS) can lead to oxidative damage, heightening vulnerability to various diseases. By contrast, ROS maintained within a moderate range plays a role in regulating normal physiological metabolism. Choosing suitable animal models in a complex research context is critical for enhancing research efficacy. While rodents are frequently utilized in medical experiments, they pose challenges such as high costs and ethical considerations. Alternatively, non-rodent model organisms like zebrafish, Drosophila, and C. elegans offer promising avenues into oxidative stress research. These organisms boast advantages such as their small size, high reproduction rate, availability for live imaging, and ease of gene manipulation. This review highlights advancements in the detection of oxidative stress using non-rodent models. The oxidative homeostasis regulatory pathway, Kelch‐like ECH‐associated protein 1-Nuclear factor erythroid 2-related factor 2 (Keap1-Nrf2), is systematically reviewed alongside multiple regulation of Nrf2-centered pathways in different organisms. Ultimately, this review conducts a comprehensive comparative analysis of different model organisms and further explores the combination of novel techniques with non-rodents. This review aims to summarize state-of-the-art findings in oxidative stress research using non-rodents and to delineate future directions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


