TMSB4Y 通过新生嘌呤合成抑制鞘磷脂合成,从而在男性食管鳞状细胞癌中发挥肿瘤抑制功能。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Y 染色体基因在癌症的性别差异中起着至关重要的作用。Y染色体基因在食管鳞状细胞癌(ESCC)中的失调和功能性影响仍未被发现。在这里,我们分析了 Y 染色体基因特征,并发现 TMSB4Y 是男性 ESCC 的一个新兴预后预测因子。功能分析显示,TMSB4Y 能抑制男性 ESCC 细胞的增殖、侵袭和转移。从机理上讲,我们证明了 TMSB4Y 与 PAICS 相互作用,TMSB4Y 会破坏 PAICS 八聚体的形成,从而抑制嘌呤的新合成,导致 AMP/ATP 比率下降,进而阻碍 AMPK 磷酸化。此外,我们还发现了一个由 TMSB4Y/PAICS-AMPK 轴协调的调控级联,它通过抑制鞘磷脂合成酶(SMSs)的表达,对鞘磷脂代谢产生抑制作用。值得注意的是,SMS1 和 SMS2 的抑制剂 Malabaricone C 能有效抑制男性 ESCC 细胞的增殖和异种移植肿瘤的生长。总之,这些发现揭示了TMSB4Y/PAICS-AMPK轴对鞘磷脂代谢的调控,并强调了靶向SMSs作为治疗男性ESCC的一种有前景的治疗方法的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
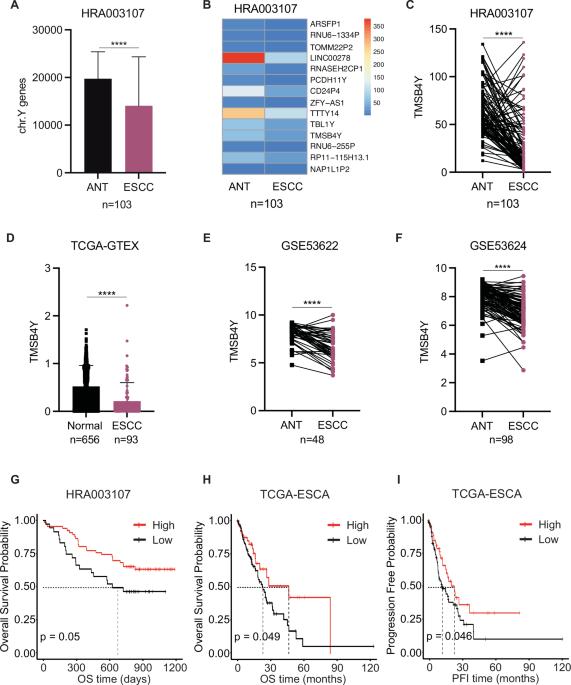
TMSB4Y restrains sphingomyelin synthesis via de novo purine synthesis to exert a tumor suppressor function in male esophageal squamous cell carcinoma
Y chromosome genes play a vital role in sex difference of cancer. The dysregulation and functional implications of Y chromosome genes in esophageal squamous cell carcinoma (ESCC) remains elusive. Here, we analyze the Y chromosome gene signature and identify TMSB4Y as an emerging prognostic predictor in male ESCC. Functional analyses show that TMSB4Y inhibits the proliferation, invasion and metastasis of male ESCC cells. Mechanistically, we demonstrate that TMSB4Y interacts with PAICS, wherein TMSB4Y disrupts the formation of the PAICS octamer to inhibit purine de novo synthesis, leading to a decrease in the AMP/ATP ratio, subsequently impeding AMPK phosphorylation. Furthermore, we uncover a regulatory cascade orchestrated by the TMSB4Y/PAICS-AMPK axis, which exerts a suppressive effect on sphingomyelin metabolism by inhibiting the expression of sphingomyelin synthases (SMSs). Notably, Malabaricone C, an inhibitor of SMS1 and SMS2, effectively suppresses male ESCC cell proliferation and xenograft tumor growth. Collectively, these findings reveal the regulation of sphingomyelin metabolism by TMSB4Y/PAICS-AMPK axis and underscore the potential of targeting SMSs as a promising therapeutic approach for the treatment of male ESCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


