ZBED3 通过 CREB 信号促进肝脏葡萄糖生成,从而加剧高血糖。
IF 10.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
背景:肝糖生成(HGP)升高是糖尿病患者肝糖代谢受损的一个突出表现。肝糖生成增加在肝糖代谢失调中起着关键作用,是导致糖尿病患者空腹高血糖的重要原因。以往的研究发现,含锌指BED结构域3(ZBED3)是2型糖尿病(T2DM)的风险基因,其单核苷酸多态性(SNPs)与空腹血糖水平密切相关,提示ZBED3与糖尿病发病之间存在潜在的相关性。本研究主要探讨ZBED3对肝糖原生成的影响,并分析调控肝糖原生成的相关信号通路:方法:评估胰岛素抵抗(IR)相关疾病患者肝脏中ZBED3的表达水平。方法:评估 ZBED3 在胰岛素抵抗(IR)相关疾病的肝脏中的表达水平,采用 RNA-seq 和生物信息学分析来研究调节 HGP 的 ZBED3 相关通路。为了研究ZBED3在肝糖原生成中的作用,研究人员使用腺相关病毒(AAV)通过上调或沉默ZBED3在小鼠原代肝细胞(MPHs)和HHL-5细胞中的表达。在体内,产生了肝细胞特异性 ZBED3 基因敲除小鼠。此外,还利用 AAV8 在 C57BL/6 和 db/db 小鼠中实现了肝细胞特异性 ZBED3 的过表达和基因敲除。免疫沉淀和质谱分析(IP-MS)被用来鉴定与ZBED3相互作用的蛋白质。通过共免疫沉淀(co-IP)、谷胱甘肽 S-转移酶(GST)下拉和双荧光素酶报告实验进一步阐明了 ZBED3 调节肝糖原生成的内在机制:结果:发现ZBED3在红外相关疾病模型肝脏中的表达增加。在胰高血糖素的刺激下,ZBED3能促进肝糖生成相关基因PGC1A、PCK1、G6PC的表达,从而增加HGP。同样,研究发现肝细胞特异性过表达 ZBED3 的小鼠肝糖原生成率升高,而 ZBED3 基因敲除的小鼠肝糖原生成率降低。此外,在 db/db 小鼠肝脏中敲除 ZBED3 会导致肝糖生成减少。此外,研究还发现,ZBED3促进了蛋白精氨酸甲基转移酶5(PRMT5)的核转位,从而影响了PRMT5介导的环磷酸腺苷(cAMP)反应元件结合蛋白(CREB)的精氨酸对称二甲基化(s-DMA)的调控,进而影响了CREB的磷酸化,最终促进了HGP的发生:本研究表明,ZBED3 促进肝糖原生成,是糖尿病进展的关键调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
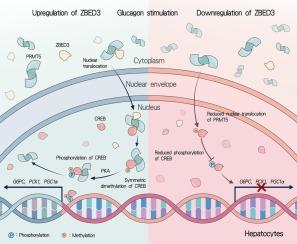
ZBED3 exacerbates hyperglycemia by promoting hepatic gluconeogenesis through CREB signaling
Background
Elevated hepatic glucose production (HGP) is a prominent manifestation of impaired hepatic glucose metabolism in individuals with diabetes. Increased hepatic gluconeogenesis plays a pivotal role in the dysregulation of hepatic glucose metabolism and contributes significantly to fasting hyperglycemia in diabetes. Previous studies have identified zinc-finger BED domain-containing 3 (ZBED3) as a risk gene for type 2 diabetes (T2DM), and its single nucleotide polymorphism (SNPs) is closely associated with the fasting blood glucose level, suggesting a potential correlation between ZBED3 and the onset of diabetes. This study primarily explores the effect of ZBED3 on hepatic gluconeogenesis and analyzes the relevant signaling pathways that regulate hepatic gluconeogenesis.
Methods
The expression level of ZBED3 was assessed in the liver of insulin-resistant (IR)-related disease. RNA-seq and bioinformatics analyses were employed to examine the ZBED3-related pathway that modulated HGP. To investigate the role of ZBED3 in hepatic gluconeogenesis, the expression of ZBED3 was manipulated by upregulation or silencing using adeno-associated virus (AAV) in mouse primary hepatocytes (MPHs) and HHL-5 cells. In vivo, hepatocyte-specific ZBED3 knockout mice were generated. Moreover, AAV8 was employed to achieve hepatocyte-specific overexpression and knockdown of ZBED3 in C57BL/6 and db/db mice. Immunoprecipitation and mass spectrometry (IP-MS) analyses were employed to identify proteins that interacted with ZBED3. Co-immunoprecipitation (co-IP), glutathione S-transferase (GST) - pulldown, and dual-luciferase reporter assays were conducted to further elucidate the underlying mechanism of ZBED3 in regulating hepatic gluconeogenesis.
Results
The expression of ZBED3 in the liver of IR-related disease models was found to be increased. Under the stimulation of glucagon, ZBED3 promoted the expression of hepatic gluconeogenesis-related genes PGC1A, PCK1, G6PC, thereby increasing HGP. Consistently, the rate of hepatic gluconeogenesis was found to be elevated in mice with hepatocyte-specific overexpression of ZBED3 and decreased in those with ZBED3 knockout. Additionally, the knockdown of ZBED3 in the liver of db/db mice resulted in a reduction in hepatic gluconeogenesis. Moreover, the study revealed that ZBED3 facilitated the nuclear translocation of protein arginine methyltransferases 5 (PRMT5) to influence the regulation of PRMT5-mediated symmetrical dimethylation of arginine (s-DMA) of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), which in turn affects the phosphorylation of CREB and ultimately promotes HGP.
Conclusions
This study indicates that ZBED3 promotes hepatic gluconeogenesis and serves as a critical regulator of the progression of diabetes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


