CDK14受IGF2BP2调控,在体外通过Wnt/β-catenin信号通路参与成骨分化。
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
目的:细胞周期蛋白依赖性激酶(CDK)家族蛋白通过调节细胞周期参与各种细胞过程;然而,人们对它们在成骨分化和绝经后骨质疏松症期间的表达情况仍知之甚少:主要方法:我们利用生物信息学方法筛选出与胰岛素样生长因子 2 mRNA 结合蛋白 2(IGF2BP2)结合的 CDK14,并在先前研究的基础上,通过时间梯度模型和绝经后骨质疏松症小鼠模型在体外探讨了它的表达。随后,我们通过CDK14 siRNA和共价抑制剂FMF-04-159-2研究了它对成骨细胞增殖、细胞周期动力学和成骨分化的影响。此外,我们还研究了 IGF2BP2 和 CDK14 之间的相互作用。最后,我们验证了CDK14对Wnt/β-catenin通路的调控作用:我们的研究结果表明,在MC3T3-E1细胞系的成骨分化过程中,CDK14的表达模式与时间有关,随着时间的推移,CDK14的表达先是增加,然后逐渐下降。值得注意的是,CDK14的表达在绝经后骨质疏松症小鼠模型的骨组织中明显减少。CDK14 抑制改变了成骨细胞的细胞周期动力学,显著降低了细胞增殖能力,并损害了成骨分化能力。IGF2BP2与CDK14 mRNA相互作用,稳定mRNA的结构并抑制其降解。此外,CDK14还能促进低密度脂蛋白受体相关蛋白6(LRP6)和糖原合成酶激酶3β(GSK3β)的磷酸化,从而调节β-catenin的水平:这些发现进一步揭示了支配成骨细胞增殖、分化和骨质疏松症的分子机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
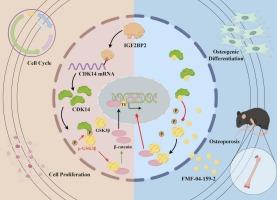
CDK14 is regulated by IGF2BP2 and involved in osteogenic differentiation via Wnt/β-catenin signaling pathway in vitro
Aims
Cyclin-dependent kinase (CDK) family proteins involve in various cellular processes via regulating the cell cycle; however, their expression during osteogenic differentiation and postmenopausal osteoporosis remains poorly understood.
Main methods
Using bioinformatics, we screened for CDK14 bound to Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) and explored its expression in vitro with time-gradient model and in a mouse model of postmenopausal osteoporosis, building on prior research. Subsequently, we investigated its effect on osteoblast proliferation, cell cycle dynamics, and osteogenic differentiation by administering CDK14 siRNA and the covalent inhibitor FMF-04-159-2. Furthermore, we examined the interaction between IGF2BP2 and CDK14. Finally, we validated the regulatory role of CDK14 on the Wnt/β-catenin pathway.
Key findings
Our findings demonstrate a time-dependent CDK14 expression patterns during osteogenic differentiation of MC3T3-E1 cell line, with an initial increase followed by gradual decline over time. Notably, CDK14 expression exhibited significant reduction in bone tissue of postmenopausal osteoporosis mouse model. CDK14 inhibition altered osteoblast cell cycle dynamics, significantly reduced cellular proliferation capacity, and impaired osteogenic differentiation ability. IGF2BP2 interacted with CDK14 mRNA, and stabilizing mRNA's structure and inhibiting its degradation. Additionally, CDK14 facilitated Low-density lipoprotein receptor-related protein 6 (LRP6) and Glycogen synthase kinase 3β (GSK3β) phosphorylation, thus regulating β-catenin levels.
Significance
These findings provide further insight into the molecular mechanisms governing osteoblast proliferation, differentiation and osteoporosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


