关于 I 型胶原蛋白糖基化在骨骼中的作用。
IF 2.7
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
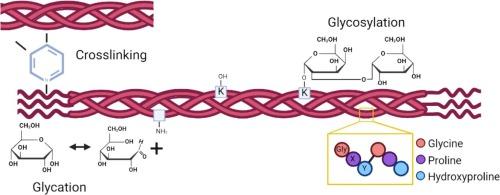
糖蛋白之间的相互作用在生物学中起着至关重要的作用,可提供诱导生化和细胞反应的额外功能。在骨骼的细胞外基质中,这种相互作用无处不在。在合成胶原分子的过程中,聚糖通过酶催化的羟基化和随后的糖基化作用被添加到特定的赖氨酸残基上。在纤维组装期间和之后,蛋白聚糖对维持组织结构、孔隙度和完整性至关重要。糖胺聚糖(GAGs)是附着在间质蛋白聚糖上的碳水化合物链,已知参与矿化。它们可以吸引和保持水分,这对骨骼的机械性能至关重要。此外,与其他长寿命蛋白质一样,胶原蛋白也容易发生糖化。胺基与葡萄糖的长期接触最终会形成高级糖化终产物(AGEs)。糖基化和糖化程度的变化已在成骨不全症和糖尿病等骨骼病变中被发现,并且似乎与骨骼质量下降有关。然而,这些变化是如何影响矿化的还不是很清楚。根据文献综述,我们假设共价连接的碳水化合物可能具有与凝胶体类似的引水功能,但在骨形成过程中的长度和时间尺度不同。糖基化可能会增加胶原三螺旋周围的水合作用,从而在水被矿物质取代后导致矿化度增加(超矿化)。同时,糖化会导致交联 AGE 的形成,这与水合水平的降低有关,从而降低了骨骼的机械性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
On the role of the glycosylation of type I collagen in bone
Glycan-protein interactions play a crucial role in biology, providing additional functions capable of inducing biochemical and cellular responses. In the extracellular matrix of bone, this type of interactions is ubiquitous. During the synthesis of the collagen molecule, glycans are post-translationally added to specific lysine residues through an enzymatically catalysed hydroxylation and subsequent glycosylation. During and after fibril assembly, proteoglycans are essential for maintaining tissue structure, porosity, and integrity. Glycosaminoglycans (GAGs), the carbohydrate chains attached to interstitial proteoglycans, are known to be involved in mineralization. They can attract and retain water, which is critical for the mechanical properties of bone. In addition, like other long-lived proteins, collagen is susceptible to glycation. Prolonged exposure of the amine group to glucose eventually leads to the formation of advanced glycation end-products (AGEs). Changes in the degree of glycosylation and glycation have been identified in bone pathologies such as osteogenesis imperfecta and diabetes and appear to be associated with a reduction in bone quality. However, how these changes affect mineralization is not well understood.
Based on the literature review, we hypothesize that the covalently attached carbohydrates may have a water-attracting function similar to that of GAGs, but at different lengths and timescales in the bone formation process. Glycosylation potentially increases the hydration around the collagen triple helix, leading to increased mineralization (hypermineralization) after water has been replaced by mineral. Meanwhile, glycation leads to the formation of crosslinking AGEs, which are associated with a decrease in hydration levels, reducing the mechanical properties of bone.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of structural biology
生物-生化与分子生物学
CiteScore
6.30
自引率
3.30%
发文量
88
审稿时长
65 days
期刊介绍:
Journal of Structural Biology (JSB) has an open access mirror journal, the Journal of Structural Biology: X (JSBX), sharing the same aims and scope, editorial team, submission system and rigorous peer review. Since both journals share the same editorial system, you may submit your manuscript via either journal homepage. You will be prompted during submission (and revision) to choose in which to publish your article. The editors and reviewers are not aware of the choice you made until the article has been published online. JSB and JSBX publish papers dealing with the structural analysis of living material at every level of organization by all methods that lead to an understanding of biological function in terms of molecular and supermolecular structure.
Techniques covered include:
• Light microscopy including confocal microscopy
• All types of electron microscopy
• X-ray diffraction
• Nuclear magnetic resonance
• Scanning force microscopy, scanning probe microscopy, and tunneling microscopy
• Digital image processing
• Computational insights into structure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


