屋尘螨 V 组过敏原拓扑结构中的核磁共振残余偶极耦合研究。
IF 2.7
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Blo t 5 是一种重要的主要过敏原蛋白,来自热带螨虫 Blomia tropicalis,这种螨虫在包括台湾在内的热带和亚热带地区非常普遍。它是一个盘卷三螺旋束,但目前对其结构折叠和三个螺旋的堆积还不清楚。我们依靠从四种不同配位介质中收集到的核磁共振残余双极耦合数据,确认 Blo t 5 具有左手螺旋拓扑结构,并进一步利用这些数据完善其溶液结构。早些时候,我们曾专门利用 TROSY NMR 实验研究了 Blo t 5 与抗体 FAB' 片段的结合,从而描述了检测单克隆抗体的构象表位。在这里,我们通过广泛的诱变和生物物理研究证实了这些发现,从而验证了我们提出的核磁共振表位图绘制方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
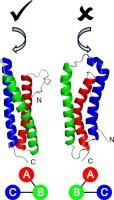
NMR residual dipolar couplings investigation in the topology of house dust mite Group V allergens
Blo t 5 is an important major allergen protein from Blomia tropicalis mites, which are prevalent in tropical and subtropical regions, including Taiwan. It is a coiled-coil triple helical bundle, but there currently is ambiguity around its structural fold and packing of the three helices. We have relied on NMR residual dipolar coupling data collected from four different alignment media to confirm that Blo t 5 has left-handed helical topology and further used that data to refine its solution structure. Earlier we had described conformational epitope for a detection monoclonal antibody by exclusive use of TROSY NMR experiments that studied Blo t 5 binding with the antibody FAB’ fragment. Here, we confirm those findings with an extensive mutagenesis and biophysical study to validate the NMR epitope mapping approach proposed by us.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of structural biology
生物-生化与分子生物学
CiteScore
6.30
自引率
3.30%
发文量
88
审稿时长
65 days
期刊介绍:
Journal of Structural Biology (JSB) has an open access mirror journal, the Journal of Structural Biology: X (JSBX), sharing the same aims and scope, editorial team, submission system and rigorous peer review. Since both journals share the same editorial system, you may submit your manuscript via either journal homepage. You will be prompted during submission (and revision) to choose in which to publish your article. The editors and reviewers are not aware of the choice you made until the article has been published online. JSB and JSBX publish papers dealing with the structural analysis of living material at every level of organization by all methods that lead to an understanding of biological function in terms of molecular and supermolecular structure.
Techniques covered include:
• Light microscopy including confocal microscopy
• All types of electron microscopy
• X-ray diffraction
• Nuclear magnetic resonance
• Scanning force microscopy, scanning probe microscopy, and tunneling microscopy
• Digital image processing
• Computational insights into structure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


