通过过滤辅助样品制备综合方法对人类羊膜间充质基质细胞分泌组进行蛋白质组分析。
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
人羊膜间充质基质细胞(hAMSCs)分泌组的免疫调节、抗炎和再生特性已得到公认,但对其特定生物活性成分的了解仍不全面。为了解决这些局限性,本研究旨在通过样品预处理、在 10 kDa 分子截断的 FASP(过滤辅助样品制备)装置上分馏以及 LC-MS 分析,对人羊膜间充质干细胞分泌组的蛋白质和肽含量进行分析。过滤保留的蛋白质部分经过胰蛋白酶消化,而未保留的则原样收集,用于完整的小蛋白质和肽分析。这种组合方法(C-FASP)可在一个步骤中收集两个互补的馏分,从而节省样品量和分析时间。对 >10 kDa 的 C-FASP 蛋白馏分进行的自下而上分析证实了我们之前的研究结果,建立了一组能一致描述 hAMSC 分泌组特征的蛋白质。对本文章由计算机程序翻译,如有差异,请以英文原文为准。
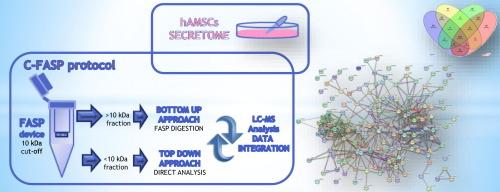
Proteomic analysis of the human amniotic mesenchymal stromal cell secretome by integrated approaches via filter-aided sample preparation
The immunomodulatory, anti-inflammatory and regenerative properties of the human amniotic mesenchymal stromal cells (hAMSCs) secretome are acknowledged but the understanding of the specific bioactive components remains incomplete. To address these limitations, the present investigation aimed to profile the proteins and peptides content of the hAMSC secretome through sample pretreatment and fractionation on 10 kDa molecular cut-off FASP (Filter Aided Sample Preparation) device and LC-MS analysis. The filter retained protein fraction underwent trypsin digestion, while the unretained was collected unchanged for intact small proteins and peptides analysis. This combined approach (C-FASP) collects in a single step two complementary fractions, advantageously saving sample volume and time of analysis. The bottom-up analysis of the C-FASP proteins fraction >10 kDa confirmed our previous findings, establishing a set of proteins consistently characterizing the hAMSC secretome. The analysis of the fraction <10 kDa, never been investigated to our knowledge, identified peptide fragments of thymosin beta 4 and beta 10, collagen alpha 1 chains I and III, alpha-enolase, and glyceraldehyde-3-phosphate dehydrogenase, involved in wound healing, anti-inflammatory response, tissue repair and regeneration, key biological activities of the secretome. C-FASP provided a comprehensive molecular profile of the hAMSC secretome offering new insights for enhanced therapeutic applications in regenerative medicine.
Significance
In this investigation we originally present the comprehensive proteomic investigation of the human amniotic mesenchymal stromal cell secretome by combining the analysis of the proteome and of the peptidome following sample pretreatment and fractionation by Filter Aided Sample Preparation (FASP) with 10 kDa molecular cut-off in coupling with LC-MS analysis. The proteome fraction retained by FASP filter was analyzed after enzymatic digestion, while the unretained fraction, below 10 kDa molecular mass, was analyzed unchanged in its intact form. This dual approach provides novel insights, previously unexplored, into the molecular components potentially responsible for the immunomodulatory and anti-inflammatory properties of the hAMSC secretome. These findings could significantly enhance the therapeutic potential of hAMSCs in regenerative medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


