新型 PD-1 抑制剂 enlonstobart(SG001)治疗 PD-L1 阳性复发性/转移性宫颈癌患者的 II 期研究。
IF 4.5
2区 医学
Q1 OBSTETRICS & GYNECOLOGY
引用次数: 0
摘要
背景:以铂为基础的化疗联合或不联合贝伐单抗是复发性或转移性宫颈癌(r/mCC)患者的一线治疗方法,但一线治疗后,r/mCC的治疗选择有限。Enlonstobart(SG001)是一种全人源化、高亲和力的抗PD-1免疫球蛋白G4单克隆抗体。之前的Ib期研究表明,SG001对PD-L1阳性的r/mCC患者有很好的疗效:在这项多中心、单臂、开放标签的II期研究中,符合条件的PD-L1阳性宫颈癌患者年龄≥18岁,一线铂类化疗进展或不耐受。患者接受每两周一次、每次 240 毫克的 SG001 治疗,持续 24 个月或直到疾病进展、出现不能耐受的毒性反应或达到其他研究终止标准。主要终点是由独立审查委员会根据 RECIST 1.1 版评估确认的客观反应率(ORR):107名患者入组,中位年龄为53岁(26-72岁)。中位随访时间为 14.0 个月(0.4-21.9 个月),确诊 ORR 为 29.0%,其中有 2 例完全应答和 29 例部分应答。疾病控制率为 54.2%。中位应答持续时间为16.6个月(95 % CI 10.8-NA),中位无进展生存期为3.1个月(95 % CI 2.2-6.9)。总生存期中位数未达到。104名患者(97.2%)出现了至少一次治疗突发不良事件TEAE,其中38名患者(35.5%)出现了3级或以上TEAE。最常见的治疗相关不良事件是白细胞减少(19.6%)、天冬氨酸氨基转移酶升高(18.7%)、贫血(17.8%)、丙氨酸氨基转移酶升高(15.9%)、甲状腺功能减退(15.0%)、中性粒细胞减少(15.0%)和甲状腺功能亢进(11.2%):结论:对于PD-L1阳性、一线铂类化疗进展或不耐受的r/mCC患者,SG001单药治疗具有持久的抗肿瘤活性和可接受的安全性:试验注册:ClinicalTrials.gov (NCT04886700)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
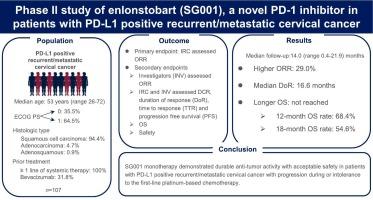
Phase II study of enlonstobart (SG001), a novel PD-1 inhibitor in patients with PD-L1 positive recurrent/metastatic cervical cancer
Background
Platinum-based chemotherapy with or without bevacizumab is the first-line treatment for patients with recurrent or metastatic cervical cancer (r/mCC), and the treatment options are limited for r/mCC after first-line treatment. Enlonstobart (SG001) is a fully humanized and high-affinity anti-PD-1 immunoglobulin G4 monoclonal antibody. Previous phase Ib study demonstrated that SG001 had a promising efficacy in patients with PD-L1 positive r/mCC.
Methods
In this multicenter, single-arm, open-label, phase II study, eligible patients were ≥ 18 years with PD-L1-positive cervical cancer who had progression on or intolerance to the first-line platinum-based chemotherapy. Patients received SG001 240 mg every two weeks for 24 months or until disease progression, intolerable toxicities, or other study discontinuation criteria were met. The primary endpoint was confirmed objective response rate (ORR) assessed by RECIST version 1.1 by independent review committee.
Results
107 patients were enrolled with median age of 53 years (range 26–72). 64.5 % of patients had a ECOG of 1. After a median follow-up of 14.0 months (range 0.4–21.9), confirmed ORR was 29.0 %, with two complete responses and twenty-nine partial responses. The disease control rate was 54.2 %. Median duration of response was 16.6 months (95 % CI 10.8-NA), median progression free survival was 3.1 months (95 % CI 2.2–6.9). Median overall survival was not reached. 104 patients (97.2 %) experienced at least one treatment emergent adverse events TEAEs, of which 38 patients (35.5 %) had grade 3 or higher TEAEs. The most common treatment-related adverse events were leukopenia (19.6 %), increased aspartate aminotransferase (18.7 %), anemia (17.8 %), increased alanine aminotransferase (15.9 %), hypothyroidism (15.0 %), neutropenia (15.0 %), and hyperthyroidism (11.2 %).
Conclusion
SG001 monotherapy demonstrated durable anti-tumor activity with acceptable safety in patients with PD-L1 positive r/mCC with progression on or intolerance to the first-line platinum-based chemotherapy.
Trial registration
ClinicalTrials.gov (NCT04886700).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gynecologic oncology
医学-妇产科学
CiteScore
8.60
自引率
6.40%
发文量
1062
审稿时长
37 days
期刊介绍:
Gynecologic Oncology, an international journal, is devoted to the publication of clinical and investigative articles that concern tumors of the female reproductive tract. Investigations relating to the etiology, diagnosis, and treatment of female cancers, as well as research from any of the disciplines related to this field of interest, are published.
Research Areas Include:
• Cell and molecular biology
• Chemotherapy
• Cytology
• Endocrinology
• Epidemiology
• Genetics
• Gynecologic surgery
• Immunology
• Pathology
• Radiotherapy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


