鞘氨醇通过激活 Nrf2、抑制泛素介导的蛋白质降解和恢复线粒体功能,减轻甲基乙二醛诱导的肌管萎缩。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
背景:甲基乙二酸(MGO)是糖应激的一种强效前体物,会导致糖尿病患者的氧化应激和肌肉萎缩。目的:本研究旨在评估 FPATM-20 对 MGO 诱导的小鼠骨骼肌 C2C12 肌细胞萎缩和线粒体功能障碍的潜在影响及其内在机制:方法:使用免疫荧光、酶联免疫吸附试验和免疫印迹法评估萎缩和抗氧化因子。线粒体功能采用 ATP 试验和海马细胞线粒体应激试验进行评估。糖原含量采用周期性酸-Schiff染色法测定。进行分子对接以确定 FPATM-20 与 Keap1 之间的相互作用:结果:在经 MGO 处理的肌管中,FPATM-20 激活了 Nrf2 通路,降低了 ROS 水平,增强了抗氧化防御能力,并提高了糖原含量。FPATM-20提高了肌管的活力和尺寸,上调了肌球蛋白重链(MyHC)的表达,调节了泛素-蛋白酶体分子(核FoxO3a、atrogin-1、MuRF-1和p62/SQSTM1),抑制了细胞凋亡(Bax/Bcl-2比率和裂解的caspase 3)。此外,FPATM-20 还能恢复线粒体功能,包括线粒体膜电位、线粒体耗氧率和线粒体生物生成途径(核 PGC-1α/TFAM/FNDC5)。用 ML385 抑制 Nrf2 可以逆转 FPATM-20 对 MGO 的影响。此外,分子对接证实了FPATM-20与Nrf2的抑制因子Keap1的结合,显示了Nrf2在保护作用中的关键作用:结论:FPATM-20通过激活Nrf2抗氧化防御、减少蛋白质降解和凋亡以及增强线粒体功能来保护肌管免受MGO毒性的伤害。因此,FPATM-20 可能是一种预防骨骼肌萎缩的新型药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
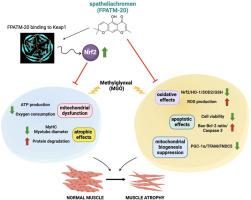
Spatheliachromen mitigates methylglyoxal-induced myotube atrophy by activating Nrf2, inhibiting ubiquitin-mediated protein degradation, and restoring mitochondrial function
Background
Methylglyoxal (MGO) is a potent precursor of glycative stress that leads to oxidative stress and muscle atrophy in diabetes. Spatheliachromen (FPATM-20), derived from Ficus pumila var. awkeotsang, exhibited potential antioxidant activity.
Purpose
This study aimed to evaluate the potential impact and underlying mechanisms of FPATM-20 on MGO-induced myotube atrophy and mitochondrial dysfunction in mouse skeletal C2C12 myotubes.
Methods
Atrophic and antioxidant factors were evaluated using immunofluorescence, enzyme-linked immunosorbent assay, and western blotting. Mitochondrial function was assessed using the ATP assay and Seahorse Cell Mito Stress Test. The glycogen content was determined using periodic acid-Schiff staining. Molecular docking was performed to determine the interaction between FPATM-20 and Keap1.
Results
In myotubes treated with MGO, FPATM-20 activated the Nrf2 pathway, reduced ROS levels, enhanced antioxidant defense, and increased glycogen content. FPATM-20 improved myotube viability and size, upregulated myosin heavy chain (MyHC) expression, modulated ubiquitin-proteasome molecules (nuclear FoxO3a, atrogin-1, MuRF-1, and p62/SQSTM1), and inhibited apoptosis (Bax/Bcl-2 ratio and cleaved caspase 3). Moreover, FPATM-20 restored mitochondrial function, including mitochondrial membrane potential, mitochondrial oxygen consumption rate, and mitochondrial biogenesis pathway (nuclear PGC-1α/TFAM/FNDC5). The inhibition of Nrf2 with ML385 reversed the effects of FPATM-20 on MGO. Furthermore, molecular docking confirmed the binding of FPATM-20 to Keap1, a suppressor of Nrf2, showing the crucial role of Nrf2 in protective effects.
Conclusions
FPATM-20 protects myotubes from MGO toxicity by activating the Nrf2 antioxidant defense, reducing protein degradation and apoptosis, and enhancing mitochondrial function. Thus, FPATM-20 may be a novel agent for preventing skeletal muscle atrophy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


