GABA 受体在麻醉和镇静中的作用:最新综述。
摘要
GABA(γ-氨基丁酸)受体是中枢神经系统中许多抑制性突触的组成成分。它们由 19 个不同亚基中的 5 个亚基组成:α1-6、β1-3、γ1-3、δ、ε、θ、π 和 ρ1-3。GABA 受体有两大亚型,即 GABAA 和 GABAB。GABAA 受体(GABAAR)由五个亚基的多种组合构成,但必须同时包含 α 和 β 亚基才能产生 GABA 门控离子通道。其他亚基包括γ、δ、ε、π和ϴ。GABAAR 有多种异构体,这些异构体决定了它们不同的亲和力和传导性等特性。作用于 GABAAR 的药物是麻醉和镇静实践的基石。用于麻醉的 GABAAR 激动剂包括异丙酚、依托咪酯、甲氧西他、硫喷妥、异氟烷、七氟烷和地氟醚。氯胺酮、氧化亚氮和氙不是 GABAR 激动剂,而是抑制谷氨酸受体(主要是 NMDA 受体)。尽管异丙酚有许多缺点,如注射时疼痛、从镇静状态快速不受控制地转变为全身麻醉以及与剂量相关的心血管抑制,但它仍然是麻醉提供者最常用的 GABAR 激动剂。此外,由于丙泊酚是以脂质乳剂配制的,因此有可能受到污染并滋生细菌。文献中充斥着各种新型异丙酚制剂,旨在解决上述诸多弊端,并取得了一定程度的成功。环糊精可与具有亲脂性的药物形成包合物,同时保持水溶性。吸入麻醉剂也是 GABA 激动剂。结合位点主要位于 α+/β- 和 β+/α- 亚基界面内,残基位于 α+/γ- 界面。异氟醚和七氟醚的结合位点可能略有不同,从而提供了意想不到的选择性。甲氧基氟烷在欧洲卷土重来,用于急诊科的快速镇痛。Penthrox(英国盖伦公司)是专门为其使用而设计的特殊装置。随着对 GABAAR 激动剂的药理学有了更深入的了解,人们开发出了更新型的镇静剂,这些镇静剂采用 "软药理学",即在产生预期治疗效果后迅速代谢为非活性代谢物的药物。这些较新的 "软 "GABAAR 激动剂具有理想镇静剂的许多特性,因为它们可以提供良好的控制、可滴定的活性和超短的作用时间。改良咪达唑仑(Remimazolam)和超短效依托咪酯类似物甲氧羰基依托咪酯(MOC-etomidate)就是其中的两个例子。甲氧羰基环丙基哌替甲酯是另一种第二代软性依托咪酯类似物,与甲氧羰基哌替甲酯相比,其效力更强,半衰期更长。此外,它可能不会导致肾上腺轴抑制。哌醋甲酯是依托咪酯的另一种软性类似物,对 11β- 羟化酶的亲和力较低,因此在临床上不太可能产生显著的肾上腺皮质抑制作用。阿法沙酮是一种 GABAAR 激动剂,最近与 7-磺丁基醚-β-环糊精(SBECD)联合配制,具有低过敏性。
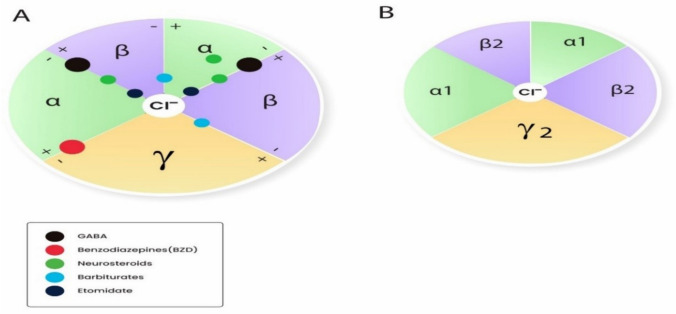
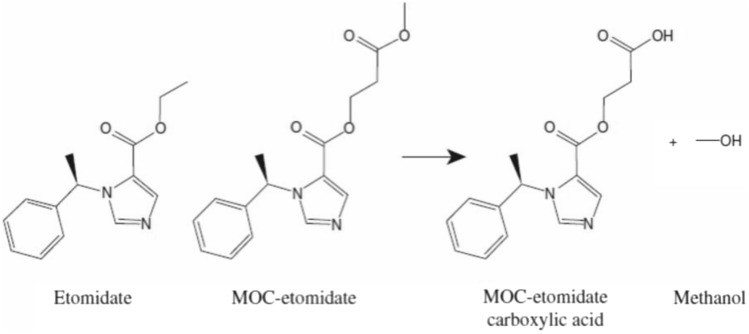
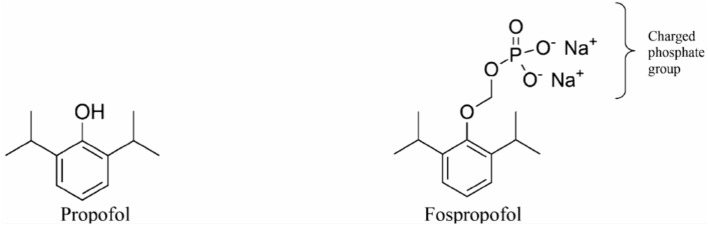
GABA (γ-aminobutyric acid) receptors are constituents of many inhibitory synapses within the central nervous system. They are formed by 5 subunits out of 19 various subunits: α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ1-3. Two main subtypes of GABA receptors have been identified, namely GABAA and GABAB. The GABAA receptor (GABAAR) is formed by a variety of combinations of five subunits, although both α and β subunits must be included to produce a GABA-gated ion channel. Other subunits are γ, δ, ε, π, and ϴ. GABAAR has many isoforms, that dictate, among other properties, their differing affinities and conductance. Drugs acting on GABAAR form the cornerstone of anesthesia and sedation practice. Some such GABAAR agonists used in anesthesia practice are propofol, etomidate, methohexital, thiopental, isoflurane, sevoflurane, and desflurane. Ketamine, nitrous oxide, and xenon are not GABAR agonists and instead inhibit glutamate receptors-mainly NMDA receptors. Inspite of its many drawbacks such as pain in injection, quick and uncontrolled conversion from sedation to general anesthesia and dose-related cardiovascular depression, propofol remains the most popular GABAR agonist employed by anesthesia providers. In addition, being formulated in a lipid emulsion, contamination and bacterial growth is possible. Literature is rife with newer propofol formulations, aiming to address many of these drawbacks, and with some degree of success. A nonemulsion propofol formulation has been developed with cyclodextrins, which form inclusion complexes with drugs having lipophilic properties while maintaining aqueous solubility. Inhalational anesthetics are also GABA agonists. The binding sites are primarily located within α+/β- and β+/α- subunit interfaces, with residues in the α+/γ- interface. Isoflurane and sevoflurane might have slightly different binding sites providing unexpected degree of selectivity. Methoxyflurane has made a comeback in Europe for rapid provision of analgesia in the emergency departments. Penthrox (Galen, UK) is the special device designed for its administration. With better understanding of pharmacology of GABAAR agonists, newer sedative agents have been developed, which utilize "soft pharmacology," a term pertaining to agents that are rapidly metabolized into inactive metabolites after producing desired therapeutic effect(s). These newer "soft" GABAAR agonists have many properties of ideal sedative agents, as they can offer well-controlled, titratable activity and ultrashort action. Remimazolam, a modified midazolam and methoxycarbonyl-etomidate (MOC-etomidate), an ultrashort-acting etomidate analog are two such examples. Cyclopropyl methoxycarbonyl metomidate is another second-generation soft etomidate analog that has a greater potency and longer half-life than MOC-etomidate. Additionally, it might not cause adrenal axis suppression. Carboetomidate is another soft analog of etomidate with low affinity for 11β-hydroxylase and is, therefore, unlikely to have clinically significant adrenocortical suppressant effects. Alphaxalone, a GABAAR agonist, is recently formulated in combination with 7-sulfobutylether-β-cyclodextrin (SBECD), which has a low hypersensitivity profile.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


