利用小分子 Wnt 拮抗剂治疗骨质疏松症。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
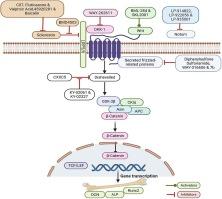
Wnt 信号是骨骼发育和平衡的关键调节因子之一。Wnt 信号调节包括干细胞命运、成骨细胞和破骨细胞活性在内的关键生物事件,从而维持骨量和骨强度。Wnt 配体是分泌型糖蛋白,可与 Frizzled(FZD)受体及其核心受体脂蛋白受体相关蛋白-5/6(LRP5/6)结合。Wnts 与 FZD 结合会触发规范(依赖于 β-catenin)和非规范(不依赖于 β-catenin)途径。在典型的 Wnt 信号传导过程中,稳定的 β-catenin 转移到细胞核,通过激活包括 Runx2 和 Osterix 在内的靶基因促进成骨细胞分化。Wnt 的负调控因子或所谓的 Wnt 拮抗剂(包括 CXXC5、sFRP、sclerostin、DKK1 和 Notum)会竞争 Fzd 的结合,从而削弱 Wnt 信号的传递。由 Wnt 信号通路突变引起的各种骨病证实了 Wnt 信号在骨稳态中的关键作用。LRP5 基因的功能缺失突变导致骨质疏松症-假性胶质瘤综合征,而功能增益突变则与以高骨密度为特征的骨化症有关。硬骨病和 Van Buchem 病是由影响 SOST 基因的突变引起的,SOST 基因编码硬骨素,是 Wnt 信号的天然抑制剂。SOST 基因的功能缺失突变会导致骨质过度生长、骨密度明显增加,以及因 Wnt 活性失控而引起的其他骨骼异常。考虑到 Wnt 信号的临床相关性,人们正在利用通过抑制内源性 Wnt 激动剂起作用的小分子来研究靶向 Wnt 抑制剂。我们采用计算生物学方法回顾了目前有关 Wnt 拮抗剂药理的数据,评估了它们作为绝经后骨质疏松症候选疗法的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Therapeutic targeting of Wnt antagonists by small molecules for treatment of osteoporosis
Wnt signaling is one of the key regulators of bone development and homeostasis. Wnt signaling regulates key biological events, including stem cell fate and osteoblast and osteoclast activity, leading to the maintenance of bone mass and strength. Wnt ligands are secreted glycoproteins that bind to Frizzled (FZD) receptors and their coreceptors, lipoprotein receptor-related proteins-5/6 (LRP5/6). Binding of Wnts to FZD triggers canonical (β-catenin-dependent) and noncanonical (β-catenin-independent) pathways. In canonical Wnt signaling, stabilized β-catenin translocates to the nucleus, where it promotes osteoblast differentiation by activating target genes, including Runx2 and Osterix. The negative regulators of Wnt or so-called Wnt antagonists, including CXXC5, sFRP, sclerostin, DKK1, and Notum, compete for Fzd binding, attenuating Wnt signaling. The critical roles of Wnt signaling in bone homeostasis have been established by various bone diseases caused by mutations in Wnt signaling pathways. Loss-of-function mutations in the LRP5 gene cause osteoporosis-pseudoglioma syndrome, whereas gain-of-function mutations are linked to osteopetrosis characterized by high bone density. Sclerosteosis and Van Buchem disease are caused by mutations affecting the SOST gene, which encodes sclerostin, a natural inhibitor of Wnt signalling. Loss-of-function mutations in SOST result in excessive bone growth, markedly increased bone density, and other skeletal abnormalities due to uncontrolled Wnt activity. Considering the clinical relevance of Wnt signaling, targeting Wnt inhibitors is being intensely pursued using small molecules that act by inhibiting endogenous Wnt agonists. We used a computational biology approach to review current data on pharmacophores of Wnt antagonists, assessing their potential as therapeutic candidates for postmenopausal osteoporosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


