克服人类胶质母细胞瘤化疗抗药性的新策略。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
替莫唑胺(TMZ)是目前治疗多形性胶质母细胞瘤(GBM)的一线化疗药物。然而,GBM固有的异质性往往导致疗效不理想,特别是对TMZ产生不同程度的耐药性。在过去的几十年中,O6-甲基鸟嘌呤-DNA 甲基转移酶(MGMT)介导的 DNA 修复通路作为克服 TMZ 耐药性的靶点已被广泛研究。然而,小分子共价 MGMT 抑制剂与 TMZ 及其他化疗药物的联合应用常常导致不良的临床效果。最近,人们发现了导致TMZ耐药的其他机制,包括表皮生长因子受体(EGFR)突变、细胞内信号通路的过度激活、能量代谢重编程或生存自噬以及肿瘤微环境(TME)的变化。这些发现表明,针对这些机制的新型治疗策略有望克服 GBM 患者的 TMZ 耐药性。在这篇综述中,我们总结了在了解 TMZ 内在和获得性耐药机制方面的最新进展。此外,我们还汇编了各种有可能减轻 GBM 化疗耐药性的小分子化合物。这些基于机制的化合物可能会提高 GBM 对 TMZ 和相关化疗药物的敏感性,从而提高临床实践中的总生存率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
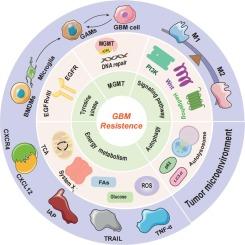
Novel strategies to overcome chemoresistance in human glioblastoma
Temozolomide (TMZ) is currently the first-line chemotherapeutic agent for the treatment of glioblastoma multiforme (GBM). However, the inherent heterogeneity of GBM often results in suboptimal outcomes, particularly due to varying degrees of resistance to TMZ. Over the past several decades, O6-methylguanine-DNA methyltransferase (MGMT)-mediated DNA repair pathway has been extensively investigated as a target to overcome TMZ resistance. Nonetheless, the combination of small molecule covalent MGMT inhibitors with TMZ and other chemotherapeutic agents has frequently led to adverse clinical effects. Recently, additional mechanisms contributing to TMZ resistance have been identified, including epidermal growth factor receptor (EGFR) mutations, overactivation of intracellular signalling pathways, energy metabolism reprogramming or survival autophagy, and changes in tumor microenvironment (TME). These findings suggest that novel therapeutic strategies targeting these mechanisms hold promise for overcoming TMZ resistance in GBM patients. In this review, we summarize the latest advancements in understanding the mechanisms underlying intrinsic and acquired TMZ resistance. Additionally, we compile various small-molecule compounds with potential to mitigate chemoresistance in GBM. These mechanism-based compounds may enhance the sensitivity of GBM to TMZ and related chemotherapeutic agents, thereby improving overall survival rates in clinical practice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


