MNAM 可提高 Blautia 的丰度并调节 Th17/Treg 的平衡,从而缓解 T2DM 小鼠的糖尿病。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究探讨了代谢衍生物 N1-甲基烟酰胺(MNAM)对高脂饮食和链脲佐菌素(STZ)诱导的 T2DM 小鼠的治疗效果,重点关注其对肠道微生物群和免疫调节的影响。MNAM 能明显降低高血糖,增强胰岛素分泌,这些效果取决于肠道微生物群的存在。它还减轻了 STZ 引起的体重减轻,改善了胰岛细胞形态,降低了胰岛细胞死亡率,提高了胰岛素(INS)水平。流式细胞术分析表明,MNAM治疗后T辅助17细胞(Th17)减少,Treg细胞增加,这与Treg标志物[白细胞介素(IL)-10、叉头盒P3(FOXP3)]上调和Th17标志物[IL17A、RAR相关孤儿受体γ(RORγt)]下调相对应。此外,MNAM 提高了抗炎 IL-10 水平,同时降低了促炎细胞因子 [IL-17α、肿瘤坏死因子 (TNF-α) 和 IL-6]。微生物组分析表明,服用 MNAM 后,微生物组的多样性减少,布劳菌丰度增加。使用布劳菌治疗不仅能逆转糖尿病指标,还能调节Th17/Treg平衡并减少炎症,其代谢产物醋酸钠通过G蛋白偶联受体43(GPR43)途径模拟这些效应。这些研究结果表明,MNAM 通过调节肠道微生物群和免疫调节来缓解糖尿病,突出了布劳提亚及其代谢物作为潜在治疗药物的作用,并为 T2DM 的新型治疗策略提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
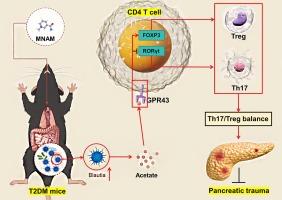
MNAM enhances Blautia abundance and modulates Th17/Treg balance to alleviate diabetes in T2DM mice
This study investigated the therapeutic effects of N1-Methylnicotinamide (MNAM), a metabolic derivative, on T2DM mice induced by a high-fat diet and streptozotocin (STZ), focusing on its impact on the gut microbiome and immune modulation. MNAM significantly reduced hyperglycemia and enhanced insulin secretion, effects that were dependent on the presence of gut microbiota. It also mitigated STZ-induced weight loss and improved islet cell morphology, reducing islet cell mortality and increasing insulin (INS) levels. Flow cytometry analysis showed a decrease in T helper 17 cells (Th17) and an increase in Treg cells after MNAM treatment, corresponding to the upregulation of Treg markers [interleukin (IL)-10, forkhead box P3 (FOXP3)] and downregulation of Th17 markers [IL17A, RAR-related orphan receptor gamma (RORγt)]. Additionally, MNAM raised anti-inflammatory IL-10 levels while reducing pro-inflammatory cytokines [IL-17α, tumor necrosis factor (TNF-α), IL-6]. Microbiome analysis revealed decreased diversity and increased Blautia abundance post-MNAM administration. Treatment with Blautia not only reversed diabetes indicators but also modulated the Th17/Treg balance and reduced inflammation, with its metabolite sodium acetate mimicking these effects through the G protein-coupled receptor 43 (GPR43) pathway. These findings suggest that MNAM’s mitigation of diabetes operates through modulation of the gut microbiota and immune regulation, highlighting Blautia and its metabolite as potential therapeutic agents and providing a theoretical foundation for novel treatment strategies in T2DM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


