miR-665/SOST 轴调控雌性小鼠骨髓间充质干细胞的表型和骨质疏松症状
IF 4.7
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
骨质疏松症是老年人,尤其是绝经后妇女常见的骨骼退化性疾病。骨髓间充质干细胞(BMSCs)是成骨细胞的祖细胞,对骨质疏松症的病理生理学至关重要。在此,我们通过基于公共数据库的生物信息学分析,锁定了在功能障碍骨髓间充质干细胞中有差异表达的miRNA。对靶 mRNA 进行了预测,并应用于信号通路和功能富集注释。研究了所选 miRNA 在体外和体内对 BMSC 增殖和成骨的影响,验证了所选 miRNA 与预测的靶 mRNA 之间的假定结合,并确定了 miRNA/mRNA 轴对 BMSC 的共同影响。与正常 BMSC 相比,骨质疏松症 BMSC 中的 miRNA 665(miR-665)下调,而在经历成骨分化的 BMSC 中则升高。在 BMSCs 中,miR-665 的过表达促进了细胞增殖和成骨分化。miR-665 靶向 Wnt 信号抑制剂硬骨蛋白(SOST),抑制了 SOST mRNA 和蛋白的表达。SOST 的过表达抑制了 BMSC 细胞的增殖和成骨分化。当与 BMSCs 共同转导时,SOST 基因敲除能显著逆转 miR-665 对 BMSCs 的影响。在卵巢切除术(OVX)诱导的骨质疏松症模型小鼠中,OVX 显著降低了骨量,而 miR-665 的过表达部分改善了 OVX 诱导的骨量丢失。总之,miR-665/SOST 轴可能通过 Wnt 信号调节小鼠 BMSC 的增殖、成骨分化和 OVX 诱导的骨质疏松症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
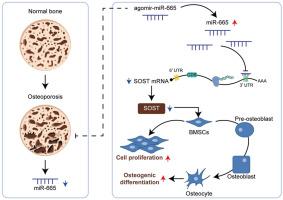
The miR-665/SOST Axis Regulates the Phenotypes of Bone Marrow Mesenchymal Stem Cells and Osteoporotic Symptoms in Female Mice
Osteoporosis is a common degenerative skeletal disease among older people, especially postmenopausal women. Bone marrow mesenchymal stem cells (BMSCs), the progenitors of osteoblasts, are essential to the pathophysiology of osteoporosis. Herein, targeting miRNAs with differential expression in dysfunctional BMSCs was accomplished by bioinformatics analysis based on public databases. Target mRNAs were predicted and applied for signaling pathway and function enrichment annotations. In vitro and in vivo effects of selected miRNA on BMSC proliferation and osteogenesis were investigated, the putative binding between selected miRNA and predicted target mRNA was verified, and the co-effects of the miRNA/mRNA axis on BMSCs were determined. miRNA 665 (miR-665) was down-regulated in osteoporotic BMSCs compared with normal BMSCs and elevated in BMSCs experiencing osteogenic differentiation. In BMSCs, miR-665 overexpression promoted cell proliferation and osteogenic differentiation. miR-665 targeted the Wnt signaling inhibitor sclerostin (SOST) and inhibited SOST mRNA and protein expression. SOST overexpression inhibited BMSC cell proliferation and osteogenic differentiation. When co-transduced to BMSCs, SOST knockdown significantly reversed the effects of miR-665 on BMSCs. In ovariectomy (OVX)-induced osteoporosis model mice, OVX remarkably decreased bone mass, whereas miR-665 overexpression partially improved OVX-induced bone mass loss. miR-665 was down-regulated in osteoporotic BMSCs and up-regulated in osteogenically differentiated BMSCs. In conclusion, the miR-665/SOST axis modulates BMSC proliferation, osteogenic differentiation, and OVX-induced osteoporosis in mice, possibly through Wnt signaling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


