非对映纯吡唑-吲哚基二氢呋喃:通过体外和硅学研究揭示异构体在抗菌作用中的选择性。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
开发用于选择性抑制细菌生长的纯非对映异构体分子混合物,为对抗日益增长的微生物抗药性开辟了新的途径。有鉴于此,我们合成了一系列非对映异构的纯吡唑基二氢呋喃(7a-7y),并利用核磁共振、液相色谱和 X 射线晶体学对其进行了表征。采用基于 DFT 的方法探讨了顺式异构体相对于反式异构体的构型稳定性。考虑到 7a 和 8a 是具有相同结构框架的代表性异构体,采用系列稀释法评估了它们对细菌和真菌菌株的生物功效差异。顺式异构体(7a)(MIC = 1.562 µg/mL)和阿莫西林(MIC = 3.125 µg/mL)对细菌生长的抑制率相对较高,这启发我们扩大底物范围,合成一系列纯非对映异构体顺式异构体作为选择性抗菌剂。然而,这两种异构体的抗真菌活性均低于研究中使用的标准药物(氟康唑)。所有反应均顺利进行,并产生了多种二氢呋喃衍生物。研究发现,所开发的合成物对小鼠成纤维细胞无细胞毒性,并且在 1 毫克/毫升的浓度下不会影响黑甘蓝种子的萌发。利用硅学分子对接和动力学研究进一步验证了实验确定的体外结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
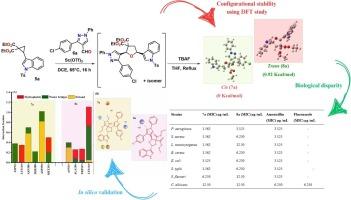
Diastereomeric pure pyrazolyl-indolyl dihydrofurans: Unveiling isomeric selectivity in antibacterial action via in vitro and in silico insights
Developing pure diastereoisomeric molecular hybrids for the selective inhibition of bacterial growth opened new avenues for combating the ever-increasing microbial resistance. Considering this, a series of diastereoisomeric pure pyrazolyl-dihydrofurans (7a-7y) were synthesized and characterized using NMR, LCMS, and X-ray crystallography. DFT based method was used to explore the configurational stability of cis over trans isomeric form. Considering 7a and 8a as representative isomeric forms with same structural framework, the difference in their bio-efficacy against bacterial and fungal strains was assessed using serial dilution method. The relatively high inhibition of bacterial growth by the cis isomeric form (7a) (MIC = 1.562 µg/mL), amoxicillin (MIC = 3.125 µg/mL) inspired us to broaden the substrate scope for synthesizing a series of pure diastereoisomeric cis forms as selective anti-bacterial agents. However, both the isomers displayed antifungal activity less than the standard drug (Fluconazole) employed in the study. All the reactions proceeded smoothly and yielded a diverse array of dihydrofuran derivatives. The developed synthetics were found to be non-cytotoxic against mouse fibroblast cells and didn’t affect the seed germination of Brassica nigra seeds when treated at 1 mg/mL concentration. The experimentally determined in vitro results were further validated using in silico molecular docking and dynamics studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


