卤代吲哚对芳基烃受体活性的调节。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
芳基烃受体(AhR)是一种由配体激活的细胞转录因子,与各种生理和病理过程密不可分。在其多种配体中,吲哚类化合物因其显著的生物活性和潜在的治疗应用而备受关注。本研究探讨了不同结构的卤代吲哚对 AhR 的激活作用。我们评估了人 LS174T-AhR-luc 报告细胞系中 AhR 的转录活性和细胞活力。在测试的化合物中,4-FI、7-FI、6-BrI、7-BrI、6-Cl-2-ox、5-Br-2-ox 和 6-Br-2-ox 以浓度依赖的方式激活 AhR,显示出较高的效力和有效性。分子对接分析表明,这些化合物与 AhR 的 PAS-B 结构域有适度的结合亲和力,竞争性放射性配体结合试验也证实了这一点。功能测定显示,卤代吲哚能诱导形成 AhR-ARNT 异源二聚体,并增强 AhR 与 CYP1A1 启动子的结合。此外,4-FI 和 7-FI 在 Caco-2 细胞模型中表现出抗炎特性,突显了它们的治疗应用潜力。这项研究强调了吲哚支架中卤素分子的类型和位置的重要性,表明它们具有开发治疗药物的潜力,可通过激活 AhR 治疗炎症性肠病等疾病。本文章由计算机程序翻译,如有差异,请以英文原文为准。
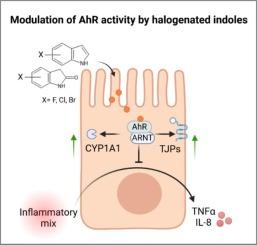
Modulation of aryl hydrocarbon receptor activity by halogenated indoles
The aryl hydrocarbon receptor (AhR) is a cytosolic ligand-activated transcription factor integral to various physiological and pathological processes. Among its diverse ligands, indole-based compounds have garnered attention due to their significant biological activity and potential therapeutic applications. This study explores the activation of AhR by structurally diverse halogenated indoles. We evaluated the transcriptional activity of AhR and cell viability in the human LS174T-AhR-luc reporter cell line. Among the tested compounds, 4-FI, 7-FI, 6-BrI, 7-BrI, 6-Cl-2-ox, 5-Br-2-ox, and 6-Br-2-ox activated AhR in a concentration-dependent manner, displaying high efficacy and potency. Molecular docking analysis revealed moderate binding affinities of these compounds to the PAS-B domain of AhR, corroborated by competitive radioligand binding assays. Functional assays showed that halogenated indoles induce the formation of AhR-ARNT heterodimer and enhance the binding of the AhR to the CYP1A1 promoter. Additionally, 4-FI and 7-FI exhibited anti-inflammatory properties in Caco-2 cell models, highlighting their potential for therapeutic applications. This study underscores the significance of the type and position of halogen moiety in indole scaffold, suggesting their potential as candidates for developing therapeutics drugs to treat conditions such as inflammatory bowel disease via AhR activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


