发现和评估新型 SHIP-1 抑制剂。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
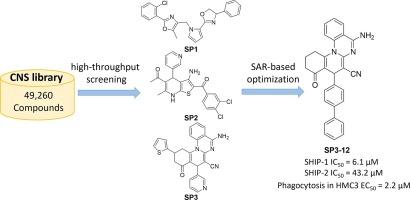
由 INPP5D 编码的 Src 同源体 2-含肌醇 5'-磷酸酶-1(SHIP-1)通过最近的遗传学和表观遗传学研究被确定为阿尔茨海默病(AD)的风险相关基因。SHIP-1 通过抑制 TREM2 级联和减少有益的微神经胶质细胞过程(包括吞噬作用)来增加阿尔茨海默病的风险。虽然有报道称几种小分子可以调节 SHIP-1 的活性,但它们在高级模型中有限的选择性和有效性限制了它们作为治疗药物或生物学研究探针的潜力。在此,我们验证并实施了一个高通量筛选平台,以探索能调节 SHIP-1 磷酸酶活性的新化学型。我们使用基于孔雀石绿的抗 SHIP-1 活性测定法筛选了 49,260 种来自商业供应商的中枢神经系统 (CNS) 蛋白化合物。通过对筛选结果的分析、优先排序和验证,我们发现了三种新型支架,它们能抑制 SHIP-1 磷酸酶的活性,IC50 低至 46.6 µM。为了提高这些有希望的新发现的抑制活性,我们进行了结构-活性关系(SAR)研究,最终发现了一种先导分子 SP3-12,它抑制 SHIP-1 的 IC50 值为 6.1 μM。SP3-12 的动力学分析表明,其抑制机制是竞争性的,对 SHIP-1 的 Ki 值为 3.2 µM,对 SHIP-2 的选择性为 7 倍。此外,在小胶质细胞吞噬/细胞健康高含量试验中的测试结果表明,SP3-12 能有效激活人小胶质细胞克隆 3 (HMC3) 细胞的吞噬功能,EC50 为 2.0 µM,在剂量范围内无细胞毒性。鉴于其效力、选择性和细胞活性,SP3-12 成为一种有潜力研究 SHIP-1 生物功能的小分子抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and evaluation of novel SHIP-1 inhibitors
Src Homology 2-containing Inositol 5′-Phosphatase-1 (SHIP-1), encoded by INPP5D, has been identified as an Alzheimer’s disease (AD) risk-associated gene through recent genetic and epigenetic studies. SHIP-1 confers AD risk by inhibiting the TREM2 cascade and reducing beneficial microglial cellular processes, including phagocytosis. While several small molecules have been reported to modulate SHIP-1 activity, their limited selectivity and efficacy in advanced models restricted their potential as therapeutic agents or probes for biological studies. Herein, we validated and implemented a high-throughput screening platform to explore new chemotypes that can modulate the phosphatase activity of SHIP-1. We screened 49,260 central nervous system (CNS)-penetrate compounds sourced from commercial vendors using the malachite green-based assay for anti-SHIP-1 activity. Through analysis, prioritization, and validation of the screening hits, we identified three novel types of scaffolds that inhibit the SHIP-1 phosphatase activity with IC50s as low as 46.6 µM. To improve the inhibitory activity of these promising hits, we carried out structure–activity relationship (SAR) studies, resulting in a lead molecule SP3-12 that inhibits SHIP-1 with an IC50 value of 6.1 μM. Kinetic analyses of SP3-12 revealed that its inhibition mechanism is competitive, with a Ki value of 3.2 µM for SHIP-1 and a 7-fold selectivity over SHIP-2. Furthermore, results from testing in a microglial phagocytosis/cell health high content assay indicated that SP3-12 could effectively activate phagocytosis in human microglial clone 3 (HMC3) cells, with an EC50 of 2.0 µM, without cytotoxicity in the dose range. Given its potency, selectivity, and cellular activity, SP3-12 emerges as a promising small molecule inhibitor with potential for investigating the biological functions of SHIP-1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


