通过立体化学配置的 4 原子碳氢化合物主链实现 310-Helix 稳定化和螺纹感应控制。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
310 螺旋是蛋白质中一种重要的二级结构,在各种蛋白质-蛋白质相互作用中发挥着至关重要的作用,然而在具有生物相关性的肽中稳定这种结构仍然具有挑战性。在本研究中,我们研究了 4 原子碳氢化合物主链稳定肽中 310 螺旋的潜力。通过闭环偏析,我们证明了主链的构型对于 310-helices的稳定和螺杆感控制至关重要。环二色性光谱显示,Ri,i+3S(4) 主链--一种在 i 位具有 (R) 构型、在 i + 3 位具有 (S) 构型、两侧有甲基的 4 原子交联--能强烈诱导右旋 310 螺旋,尤其是在含有蛋白质源 l- 氨基酸的序列中。此外,多重主链还能有效稳定较长的肽段,突出了这种方法在肽治疗和生物分子工程中应用的多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
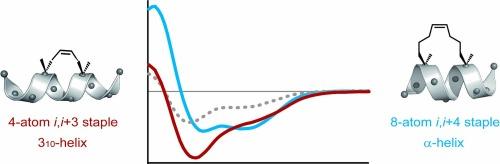
310-Helix stabilization and screw sense control via stereochemically configured 4-atom hydrocarbon staples
The 310-helix is a crucial secondary structure in proteins, playing an essential role in various protein–protein interactions, yet stabilizing it in biologically relevant peptides remains challenging. In this study, we investigated the potential of 4-atom hydrocarbon staples to stabilize 310-helices in peptides. Using ring-closing metathesis, we demonstrated that the staple’s configuration is critical for both the stabilization and screw sense control of 310-helices. Circular dichroism spectroscopy revealed that the Ri,i+3S(4) staple—a 4-atom cross-link with (R)-configuration at the i position, (S)-configuration at the i + 3 position, and flanked by methyl groups—strongly induces right-handed 310-helices, especially in sequences with proteinogenic l-amino acids. Furthermore, multiple staples effectively stabilized longer peptides, underscoring the versatility of this approach for applications in peptide therapeutics and biomolecular engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


