抗体阻断 PSGL-1 免疫检查点可增强 T 细胞对 B 细胞淋巴瘤的反应
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
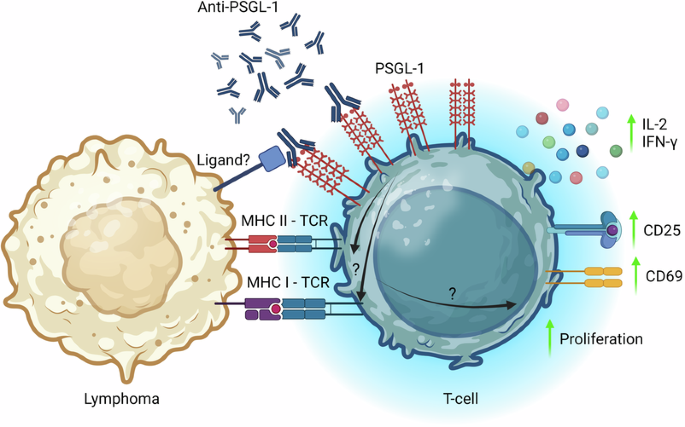
尽管癌症免疫疗法取得了进展,但大多数淋巴瘤对检查点抑制剂仍无反应。P-选择素糖蛋白配体-1(P-selectin glycoprotein ligand-1,PSGL-1)最近被确定为小鼠黑色素瘤模型中T细胞衰竭的促进因子,它已成为一种新型免疫检查点蛋白和有希望的免疫治疗靶点。在这项研究中,我们探讨了 PSGL-1 抗体靶向治疗 B 细胞淋巴瘤的潜力。利用异体共培养系统,我们证明了针对人 PSGL-1 的靶向抗体干预能增强 T 细胞的活化和效应细胞因子的产生,以应对淋巴瘤细胞。此外,用 PSGL-1 抗体体外处理原代淋巴瘤细胞悬浮液可增强自体淋巴瘤浸润 T 细胞的活化。通过使用 A20 合成 B 细胞淋巴瘤小鼠模型,我们发现 PSGL-1 抗体治疗能显著减缓肿瘤的发展并减少终点肿瘤负荷。这种抗肿瘤效应伴随着 CD4+ 和 CD8+ T 细胞的肿瘤浸润增加和调节性 T 细胞浸润的减少。最后,服用抗 PSGL-1 能增强先前转移到携带侵袭性 Eμ-Myc 淋巴瘤细胞小鼠体内的 CAR T 细胞的扩增,并改善疾病控制。这些结果表明,PSGL-1 抗体阻断增强了 T 细胞对抗 B 细胞淋巴瘤的活性,为治疗这些恶性肿瘤提供了一种潜在的新型免疫治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Antibody blockade of the PSGL-1 immune checkpoint enhances T-cell responses to B-cell lymphoma
Despite advancements in cancer immunotherapy, most lymphomas remain unresponsive to checkpoint inhibitors. P-selectin glycoprotein ligand-1 (PSGL-1), recently identified as a promoter of T-cell exhaustion in murine melanoma models, has emerged as a novel immune checkpoint protein and promising immunotherapeutic target. In this study, we investigated the potential of PSGL-1 antibody targeting in B-cell lymphoma. Using allogeneic co-culture systems, we demonstrated that targeted antibody interventions against human PSGL-1 enhanced T-cell activation and effector cytokine production in response to lymphoma cells. Moreover, in vitro treatment of primary lymphoma cell suspensions with PSGL-1 antibody resulted in increased activation of autologous lymphoma-infiltrating T cells. Using the A20 syngeneic B-cell lymphoma mouse model, we found that PSGL-1 antibody treatment significantly slowed tumor development and reduced the endpoint tumor burden. This antitumoral effect was accompanied by augmented tumor infiltration of CD4+ and CD8+ T cells and reduced infiltration of regulatory T cells. Finally, anti-PSGL-1 administration enhanced the expansion of CAR T cells previously transferred to mice bearing the aggressive Eμ-Myc lymphoma cells and improved disease control. These results demonstrate that PSGL-1 antibody blockade bolsters T-cell activity against B-cell lymphoma, suggesting a potential novel immunotherapeutic approach for treating these malignancies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


