关于数字病理学人工智能产品的公开证据
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
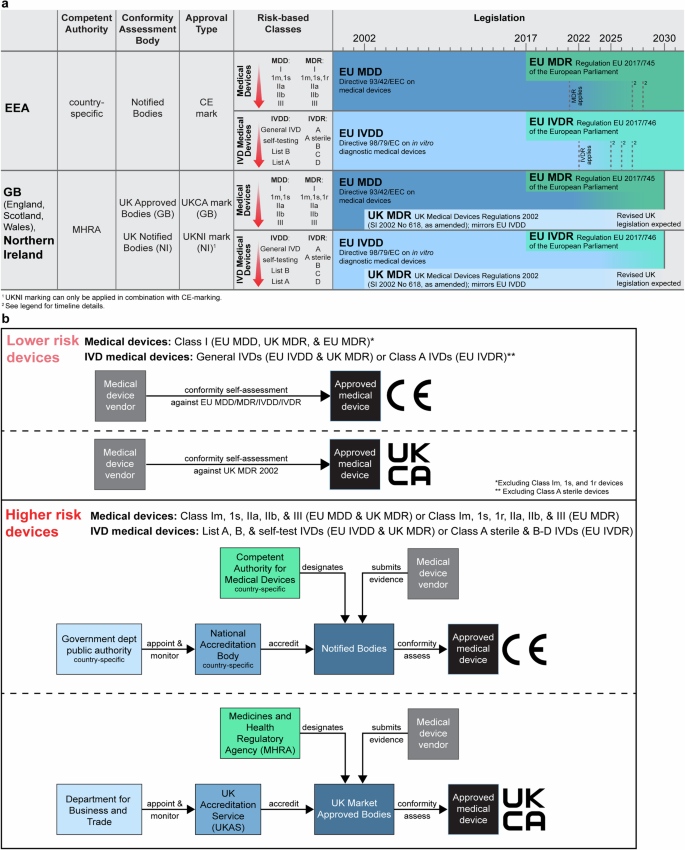
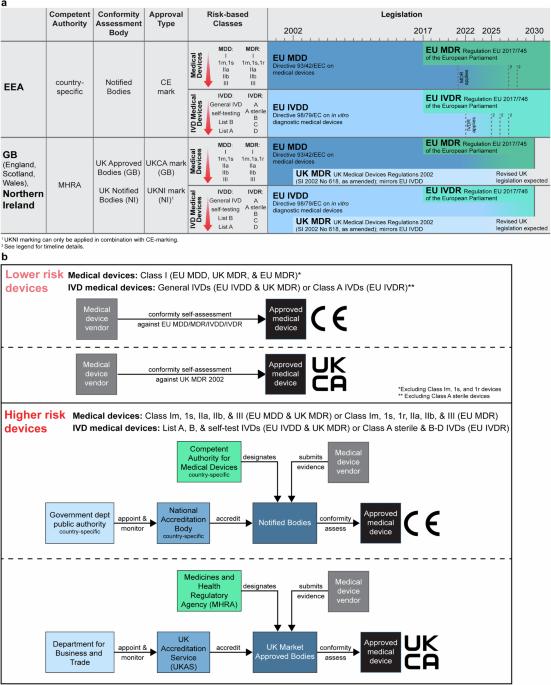
将基于人工智能(AI)的方法应用于数字病理图像的新型产品被吹捧为具有多种用途和优势。然而,有关产品的公开信息可能不尽相同,独立证据的来源也很少。本综述旨在确定基于人工智能的数字病理学产品的公开证据。审查了欧洲经济区/大不列颠(EEA/GB)市场上产品的主要特征,包括其监管批准、预期用途和已发表的验证研究。符合纳入标准的人工智能产品共有 26 种,其中 24 种已通过自我认证途径获得监管部门批准,成为通用体外诊断 (IVD) 医疗设备。其中只有 10 种产品(38%)进行了同行评审的内部验证研究,11 种产品(42%)进行了同行评审的外部验证研究。为了提高透明度,我们利用已确认的公共证据开发了一个在线登记册 (https://osf.io/gb84r/),预计该登记册将提供有关新型器械的可访问资源,并为决策提供支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Public evidence on AI products for digital pathology
Novel products applying artificial intelligence (AI)-based methods to digital pathology images are touted to have many uses and benefits. However, publicly available information for products can be variable, with few sources of independent evidence. This review aimed to identify public evidence for AI-based products for digital pathology. Key features of products on the European Economic Area/Great Britain (EEA/GB) markets were examined, including their regulatory approval, intended use, and published validation studies. There were 26 AI-based products that met the inclusion criteria and, of these, 24 had received regulatory approval via the self-certification route as General in vitro diagnostic (IVD) medical devices. Only 10 of the products (38%) had peer-reviewed internal validation studies and 11 products (42%) had peer-reviewed external validation studies. To support transparency an online register was developed using identified public evidence ( https://osf.io/gb84r/ ), which we anticipate will provide an accessible resource on novel devices and support decision making.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


