I2 促进三种不同胺类的氧化环化,以获得多种生物杂芳基
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用叔烷基胺作为可靠的双碳构建模块,建立了一条从 2-甲基喹啉和芳基胺区域选择性合成生物喹啉的简洁而高效的路线。这种 I2 促进的方法通过两种瞬时烯胺实现了三种亲核物质的 [4π + 2σ] 环化。C(sp3)-H 环化策略还扩展到了其他甲基氮杂烯烃,从而构建出了非对称的含苯并[f]喹啉的双杂芳基。值得注意的是,复杂分子的后期功能化赋予了这一过程在生物相关领域的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
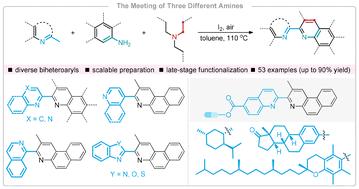
I2-Promoted oxidative annulation of three different amines to access diverse biheteroaryls†
Utilizing tertiary alkylamines as reliable two-carbon building blocks, a concise and efficient route for the regioselective synthesis of biquinolines from 2-methylquinolines and arylamines has been established. This I2-promoted approach enables a [4π + 2σ] annulation of three nucleophilic species via two kinds of transient enamines. The C(sp3)–H annulation strategy is also extended to other methylazaarenes toward constructing nonsymmetrical benzo[f]quinoline-containing biheteroaryls. Notably, the applicability of this process for the late-stage functionalization of complex molecules highlights its considerable potential for application in biorelevant areas.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


