研究 BP 热分解为 B12P2:高温下的实验启示和动力学建模
IF 8.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
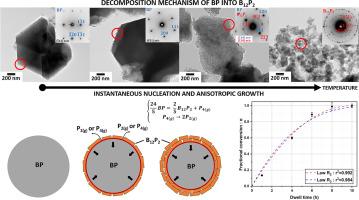
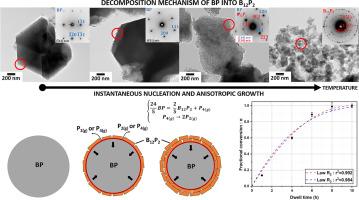
本研究探讨了 BP 热分解为 B12P2 的过程,尤其关注通过自蔓延高温反应合成的 BP 粉末的异质固气机理。研究结合了 XRD、TGA、SEM、TEM、EELS 和 SAED 等多种技术,考察了不同温度下的形态和结构变化。结果显示,分解的点火温度为 1120°C,超过这一温度,BP 晶粒会碎裂成更小的颗粒,同时释放出磷气体(P2(g)、P4(g))。此外,对热处理过的粉末进行里特维尔德细化,有助于确定不同等温条件下分解反应的推进率值。根据这些在 1300°C 下获得的动力学数据,建立了一个具有瞬时成核和各向异性生长的单过程成核-生长模型。特别是,该模型描述了球形晶粒向内发展的过程,以及在内部界面发生的反应作为决定速率的步骤,显示出很强的相关系数,为 BP 向 B12P2 转化的动力学演变提供了新的见解。这些结果有助于加深对热分解过程及其基本机制的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigating the thermal decomposition of BP into B12P2: experimental insights and kinetic modelling at high temperatures
This study investigates the thermal decomposition of BP into B12P2, with a particular focus on the heterogeneous solid-gas mechanism involving BP powders synthesized via self-propagating high temperature reactions. Various techniques, including XRD, TGA, SEM, TEM, EELS and SAED, were combined to examine morphological and structural changes at different temperatures. The results reveal an ignition temperature for decomposition at 1120 °C, beyond which BP grains undergo fragmentation into smaller particles, accompanied by the release of phosphorous gases (P2(g), P4(g)). Additionally, Rietveld refinements on thermally treated powders facilitated the determination of advancement rate values of the decomposition reaction under different isothermal conditions. Based on these kinetic data obtained at 1300 °C, a one-process nucleation-growth model with instantaneous nucleation and anisotropic growth has be established. In particular, the law, which describes the process for a spherical grain with inward development and a reaction occurring at the internal interface as the step determining the rate, displayed a strong correlation coefficient, offering novel insights into the kinetic evolution of the BP to B12P2 conversion. These results contribute to a deeper understanding of the thermal decomposition process and its underlying elementary mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Materialia
工程技术-材料科学:综合
CiteScore
16.10
自引率
8.50%
发文量
801
审稿时长
53 days
期刊介绍:
Acta Materialia serves as a platform for publishing full-length, original papers and commissioned overviews that contribute to a profound understanding of the correlation between the processing, structure, and properties of inorganic materials. The journal seeks papers with high impact potential or those that significantly propel the field forward. The scope includes the atomic and molecular arrangements, chemical and electronic structures, and microstructure of materials, focusing on their mechanical or functional behavior across all length scales, including nanostructures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


