NPM1 融合蛋白通过 XPO1 依赖性 HOX 激活促进髓系白血病的发生
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
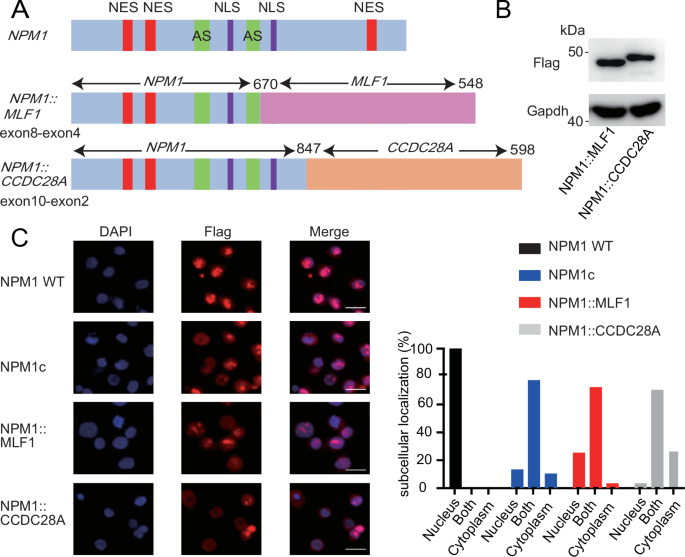
Nucleophosmin(NPM1)是一种核仁蛋白,也是急性髓性白血病(AML)中最常发生突变的基因之一。除了常见的外显子12(NPM1c)框移突变外,先前的研究还发现了导致小儿急性髓性白血病中NPM1融合蛋白表达的NPM1基因重排。然而,NPM1融合蛋白是否真的致癌以及NPM1融合蛋白是如何导致急性髓细胞性白血病的一直是个未知数。在本研究中,我们研究了两种罕见的NPM1融合蛋白--NPM1::MLF1和NPM1::CCDC28A--的亚细胞定位和致白血病潜能。NPM1::MLF1 同时存在于细胞核和细胞质中,在小鼠移植试验中偶尔会诱发急性髓细胞白血病。NPM1::CCDC28A更多地定位于细胞质,能在体外使小鼠骨髓细胞永生,并在体内有效诱导急性髓细胞性白血病。从机理上讲,两种 NPM1 融合体都与 HOX 基因簇结合,并且与 NPM1c 一样,会与 XPO1 合作导致 HOX 基因异常上调。XPO1 抑制剂 selinexor 可抑制 NPM1 融合体驱动的 HOX 激活和集落形成。NPM1::CCDC28A细胞对menin抑制也很敏感。因此,我们的研究提供了实验证据,证明NPM1::MLF1和NPM1::CCDC28A都是致癌基因,其功能与NPM1c相似。抑制 XPO1 和 menin 可能是治疗 NPM1 重组急性髓细胞性白血病的一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

NPM1-fusion proteins promote myeloid leukemogenesis through XPO1-dependent HOX activation
Nucleophosmin (NPM1) is a nucleolar protein and one of the most frequently mutated genes in acute myeloid leukemia (AML). In addition to the commonly detected frameshift mutations in exon12 (NPM1c), previous studies have identified NPM1 gene rearrangements leading to the expression of NPM1-fusion proteins in pediatric AML. However, whether the NPM1-fusions are indeed oncogenic and how the NPM1-fusions cause AML have been largely unknown. In this study, we investigated the subcellular localization and leukemogenic potential of two rare NPM1-fusion proteins, NPM1::MLF1 and NPM1::CCDC28A. NPM1::MLF1 is present in both the nucleus and cytoplasm and occasionally induces AML in the mouse transplantation assay. NPM1::CCDC28A is more localized to the cytoplasm, immortalizes mouse bone marrow cells in vitro and efficiently induces AML in vivo. Mechanistically, both NPM1-fusions bind to the HOX gene cluster and, like NPM1c, cause aberrant upregulation of HOX genes in cooperation with XPO1. The XPO1 inhibitor selinexor suppressed HOX activation and colony formation driven by the NPM1-fusions. NPM1::CCDC28A cells were also sensitive to menin inhibition. Thus, our study provides experimental evidence that both NPM1::MLF1 and NPM1::CCDC28A are oncogenes with functions similar to NPM1c. Inhibition of XPO1 and menin may be a promising strategy for the NPM1-rearranged AML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


