扑热息痛通过竞争性抑制和清除作用抑制中性粒细胞氧自由基。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
中性粒细胞是先天性和适应性免疫反应的关键细胞,它们利用活性氧(ROS)来对抗病原体和控制基因表达。扑热息痛(对乙酰氨基酚)被广泛用作镇痛和解热药物,但其确切的作用机制尚未完全清楚。在此,我们利用流式细胞仪和抗氧化测定法研究了摄入和体外对乙酰氨基酚对中性粒细胞 ROS 活性的影响。我们的研究表明,扑热息痛能在短期内明显抑制体外 ROS 活性。此外,扑热息痛及其代谢物 N-acetyl-p-benzoquinone imine 都表现出直接的体外抗氧化作用,扑热息痛还能抑制体内中性粒细胞胞外陷阱的形成。这些发现表明,扑热息痛的使用与中性粒细胞反应的改变之间存在联系,对某些患者群体(如免疫力低下者)的使用具有潜在影响。有必要进一步研究扑热息痛对中性粒细胞抗菌功能的影响,以阐明可能存在的风险,尤其是在频繁服用或与疫苗接种等其他治疗同时使用时。本文章由计算机程序翻译,如有差异,请以英文原文为准。
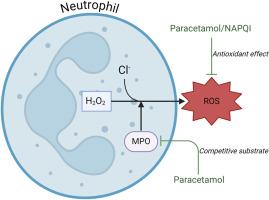
Paracetamol suppresses neutrophilic oxygen radicals through competitive inhibition and scavenging
Neutrophils, pivotal cells of innate and adaptive immune responses, employ reactive oxygen species (ROS) to combat pathogens and control gene expression. Paracetamol (acetaminophen) is widely used as an analgesic and antipyretic medication, yet its precise mechanisms of action are not yet fully understood. Here, we investigate the impact of both ingested and in-vitro paracetamol on neutrophil ROS activity, using flow cytometry and antioxidant assays. Our studies reveal that paracetamol significantly suppresses ROS activity ex-vivo in the short term. Additionally, both paracetamol and its metabolite N-acetyl-p-benzoquinone imine exhibited direct in vitro antioxidant effects, and paracetamol suppressed neutrophil extracellular trap formation ex vivo. These findings suggest a connection between paracetamol use and altered neutrophil responses, with potential implications for use in some patient groups, such as immunocompromised individuals. Further investigation into paracetamol's effects on neutrophil antimicrobial functions is warranted to elucidate possible risks, particularly when taken frequently or in conjunction with other treatments such as vaccinations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


