AKR1B10在间充质干细胞成骨分化和萎缩性骨不连中的作用。
IF 3.5
2区 医学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
萎缩性骨不连是一种没有有效药物治疗的慢性疾病。本文采用高通量 mRNA 测序技术探索萎缩性骨不连的新靶点。AKR1B10是醛酮还原酶家族1的成员,在萎缩性骨不连组织中上调。目前还没有研究揭示 AKR1B10 在萎缩性骨不连中的作用。我们利用大鼠骨髓间充质干细胞(BMSCs)探讨了AKR1B10对成骨分化和自噬的影响。在体内,我们将负载有 LV-shAKR1B10 转导的 BMSCs 的胶原海绵植入大鼠骨折的股骨中,以探索 AKR1B10 在骨折愈合中的作用。结果表明,AKR1B10能降低ALP的活性,抑制COL1A1、RUNX2和OCN的表达,抑制骨分化BMSCs的钙化沉积。AKR1B10 可降低 LC3II 的表达,减少自噬体的数量,促进 p62 的表达。此外,AKR1B10 基因敲除对 BMSCs 成骨分化的促进作用会因 3-MA 处理而减弱。植入胶原海绵后发现,敲除 AKR1B10 能促进骨折愈合。总之,AKR1B10 可抑制成骨分化和自噬,延缓骨折愈合。这些结果为揭示 AKR1B10 在骨不连中的作用提供了一个新的视角,也为治疗骨不连提供了一个新的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
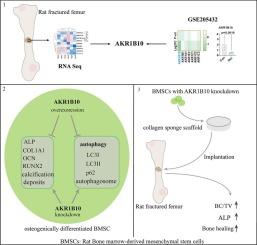
The role of AKR1B10 in osteogenic differentiation of mesenchymal stem cells and atrophic nonunion
Atrophic nonunion is a chronic disease without effective medications. Here, high-throughput mRNA sequencing was used to explore the novel targets in atrophic nonunion. AKR1B10, a member of aldo-keto reductase family 1, is upregulated in atrophic nonunion tissues. There are currently no studies to reveal the role of AKR1B10 in atrophic nonunion. We used rat bone marrow-derived mesenchymal stem cells (BMSCs) to explore the effect of AKR1B10 on the osteogenic differentiation and autophagy. In vivo, we implanted collagen sponges loaded with LV-shAKR1B10-transduced BMSCs into rat fractured femurs to explore the role of AKR1B10 in fracture healing. The results showed that AKR1B10 reduced the activity of ALP, suppressed the expression of COL1A1, RUNX2 and OCN, and inhibited calcification deposition in osteogenically differentiated BMSCs. AKR1B10 reduced the expression of LC3II, decreased the number of autophagosomes, and promoted the expression of p62. In addition, the promoting effect of AKR1B10 knockdown on osteogenic differentiation of BMSCs was attenuated by 3-MA treatment. Implantation of collagen sponges found that knockdown of AKR1B10 promoted bone fracture healing. In conclusion, AKR1B10 inhibited the osteogenic differentiation and autophagy, and delayed the bone fracture healing. These results provide a new perspective on revealing the role of AKR1B10 in nonunion and may also provide a new therapeutic target for the treatment of nonunion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bone
医学-内分泌学与代谢
CiteScore
8.90
自引率
4.90%
发文量
264
审稿时长
30 days
期刊介绍:
BONE is an interdisciplinary forum for the rapid publication of original articles and reviews on basic, translational, and clinical aspects of bone and mineral metabolism. The Journal also encourages submissions related to interactions of bone with other organ systems, including cartilage, endocrine, muscle, fat, neural, vascular, gastrointestinal, hematopoietic, and immune systems. Particular attention is placed on the application of experimental studies to clinical practice.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


