NEU4 介导的脱ialylation 可增强卵巢癌扩散过程中致癌受体的激活。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
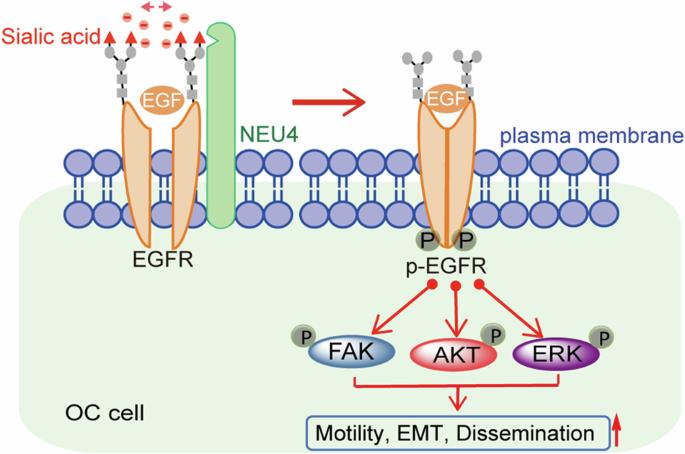
糖基化深刻影响着卵巢癌(OC)腹膜播散转移过程中癌细胞与微环境基质细胞之间的相互作用,而卵巢癌是癌症相关死亡的主要原因。尽管特征性癌症糖结合物被广泛用作癌症诊断的生物标记物,但由于分析具有巨大结构和功能异质性的糖链的技术限制,我们对癌症糖基因组的了解仍然相当零散。鉴于糖基化机制的改变定义了失调的癌症糖粒结构,我们在此对参与糖基化的 498 个基因进行了系统的功能缺失筛选,以寻找 OC 传播的关键调控因子。我们发现了神经氨酸酶 4(NEU4),它是一种能够水解糖共轭物中末端sialic酸的酶,是促进腹膜扩散的OC糖蛋白的重要调节因子。在人类高级别浆液性卵巢癌(HGSOC)患者中,与原发肿瘤细胞相比,在扩散的卵巢癌细胞中检测到 NEU4 增高,这与患者的生存率显著相关。在人NEU4的三种替代剪接生成的同工酶中,我们发现只有质膜定位的NEU4同工酶2(NEU4-iso2)和胞内同工酶3通过增强细胞运动和上皮-间质转化促进了OC的腹膜扩散。我们还鉴定了NEU4-iso2调控的细胞表面糖蛋白组,发现NEU4-iso2对上皮生长因子受体(EGFR)进行了去氨酰化,尤其是在N196残基上,从而使EGFR及其下游促肿瘤信号级联过度激活。我们的研究结果为了解 OC 进展过程中 OC 糖粒如何失调提供了新的视角,并揭示了表皮生长因子受体在癌症中异常激活的一个重要功能糖基复合体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
NEU4-mediated desialylation enhances the activation of the oncogenic receptors for the dissemination of ovarian carcinoma
Glycosylation profoundly influences the interactions between cancer cells and microenvironmental stromal cells during the peritoneal disseminated metastasis of ovarian carcinoma (OC), which is the major cause of cancer-related death. Although the characteristic cancer glycoconjugates are widely used as biomarkers for cancer diagnosis, our knowledge about cancer glycome remains quite fragmented due to the technique limitations in analyzing glycan chains with tremendous structural and functional heterogeneity. Given the dysregulated cancer glycome is defined by the altered glycosylation machinery, here we performed a systematic loss-of-function screen on 498 genes involved in glycosylation for key regulators of OC dissemination. We identified neuraminidase 4 (NEU4), an enzyme capable of hydrolyzing terminal sialic acid from glycoconjugates, as a vital peritoneal dissemination-promoting modifier of OC glycome. In human patients with high-grade serous OC (HGSOC), increased NEU4 was detected in the disseminated OC cells when compared with that in the primary tumor cells, which significantly correlated with the worse survival. Among three alternative splice-generated isoforms of human NEU4, we revealed that only the plasma membrane-localized NEU4 isoform 2 (NEU4-iso2) and intracellular isoform 3 promoted the peritoneal dissemination of OC by enhancing the cell motility and epithelial-mesenchymal transition. We also identified NEU4-iso2-regulated cell surface glycoproteome and found that NEU4-iso2 desialylated the epithelial growth factor receptor (EGFR), in particular at N196 residue, for the hyperactivation of EGFR and its downstream tumor-promoting signaling cascades. Our results provide new insights into how the OC glycome is dysregulated during OC progression and reveal a functionally important glycosite on EGFR for its abnormal activation in cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


