CypA/TAF15/STAT5A/miR-514a-3p反馈回路驱动卵巢癌转移。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
环嗜蛋白酶 A(CypA)是一种肽基-脯氨酰异构酶,参与多种癌症事件,但卵巢癌(OC)中 CypA 的异常表达和调控的分子机制却从未被考虑过。本研究发现CypA是卵巢癌上皮-间质转化(EMT)的关键驱动因素,并探讨了这一过程的机制。我们发现,CypA 在卵巢癌患者的组织和血清中上调,CypA 的过表达与不良预后相关。在体内皮下肿瘤异种移植和腹腔转移模型中,CypA 促进了肿瘤的生长和转移。体外研究表明,CypA 通过激活 PI3K/AKT 信号通路加速卵巢癌细胞上皮-间质转化。机制研究表明,STAT5A 结合 pri-miR-514a-3p 并抑制其活性,而 miR-514a-3p 直接结合 CypA 的 3'-UTR 抑制其表达,导致 STAT5A 促进 CypA 的表达,形成 STAT5A/miR-514a-3p/CypA 轴。此外,免疫沉淀物和质谱分析确定了 CypA 与 TAF15 的相互作用,这种相互作用通过抑制 TAF15 的蛋白酶体降解来稳定 TAF15,并促进其进入细胞核。而 STAT5A 则受到 TAF15 的正向调节。我们的研究结果为CypA确定了一个新的反馈环路,该环路通过TAF15/STAT5A/miR-514a-3p途径驱动卵巢癌的EMT和卵巢肿瘤的生长和转移,并促进CypA释放到细胞外,这为OC治疗提供了一个有希望的治疗靶点和诊断生物标志物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
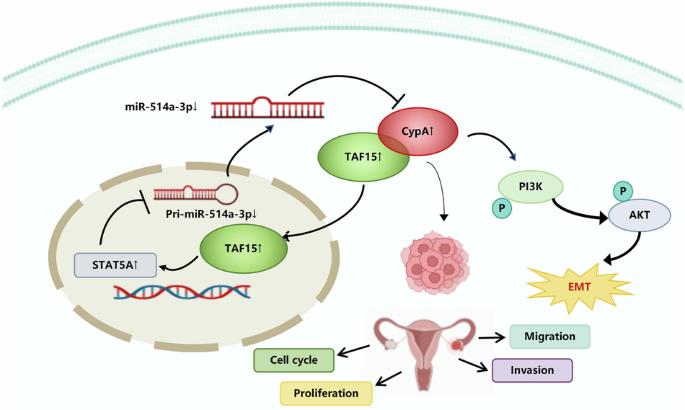
CypA/TAF15/STAT5A/miR-514a-3p feedback loop drives ovarian cancer metastasis
Cyclophilin A (CypA) is a peptidyl-prolyl isomerase that participates in multiple cancer events, but the molecular mechanisms of abnormal expression and regulation of CypA in ovarian cancer (OC) have never been considered. This study identifies CypA as a key driver of epithelial-mesenchymal transition (EMT) in ovarian cancer and explores the mechanisms that underly this process. We show that CypA is upregulated in tissues and serum of ovarian cancer patients and that CypA overexpression correlates with poor prognosis. CypA facilitates tumor growth and metastasis in vivo in subcutaneous tumor xenograft and abdominal metastatic models, and in vitro studies suggest a mechanism, showing that CypA accelerates ovarian cancer cell epithelial-mesenchymal transition by activating a PI3K/AKT signaling pathway. Mechanistic studies showed that STAT5A binds pri-miR-514a-3p and inhibits its activity, whereas miR-514a-3p directly binds to the 3ʹ-UTR of CypA to suppress its expression, resulting in STAT5A promoting the expression of CypA, forming the STAT5A/miR-514a-3p/CypA axis. Furthermore, immunoprecipitates and mass spectrometry analysis identifies a CypA interaction with TAF15 that stabilizes TAF15 by suppressing its proteasome degradation and promotes its entry into the nucleus. While STAT5A is positively regulated by TAF15. Our findings identify a novel feedback loop for CypA that drives EMT and ovarian tumor growth and metastasis via a TAF15/STAT5A/miR-514a-3p pathway in ovarian cancer and facilitates the release of CypA into the extracellular, which provides a promising therapeutic target for OC treatment and a diagnostic biomarker.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


